Inorganic solid electrolyte with surface made of amorphous substance and preparation method thereof
A technology of amorphous substances and inorganic solids, applied in circuits, electrical components, secondary batteries, etc., can solve problems such as large interface impedance, contact deterioration, hidden dangers of lithium dendrites, etc., and achieve the effect of reducing high impedance and stabilizing contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The invention provides a method for preparing an inorganic solid electrolyte whose surface is an amorphous substance, comprising the following steps:
[0060] A) Prepare an amorphous substance with the same chemical composition as the solid electrolyte matrix material by melting-quenching method or high-energy ball milling method;
[0061] B) mixing the amorphous substance, binder and solvent to obtain a composite material slurry;
[0062] C) coating the composite material slurry on the surface of the solid electrolyte matrix material, removing the solvent and binder and softening the amorphous substance to obtain an inorganic solid electrolyte with an amorphous substance on the surface.
[0063] The invention adopts a melting-quenching method or a high-energy ball milling method to prepare the amorphous substance with the same chemical composition as the solid electrolyte matrix material.
[0064] Wherein, the melting-quenching method is:
[0065] The solid electroly...
Embodiment 1
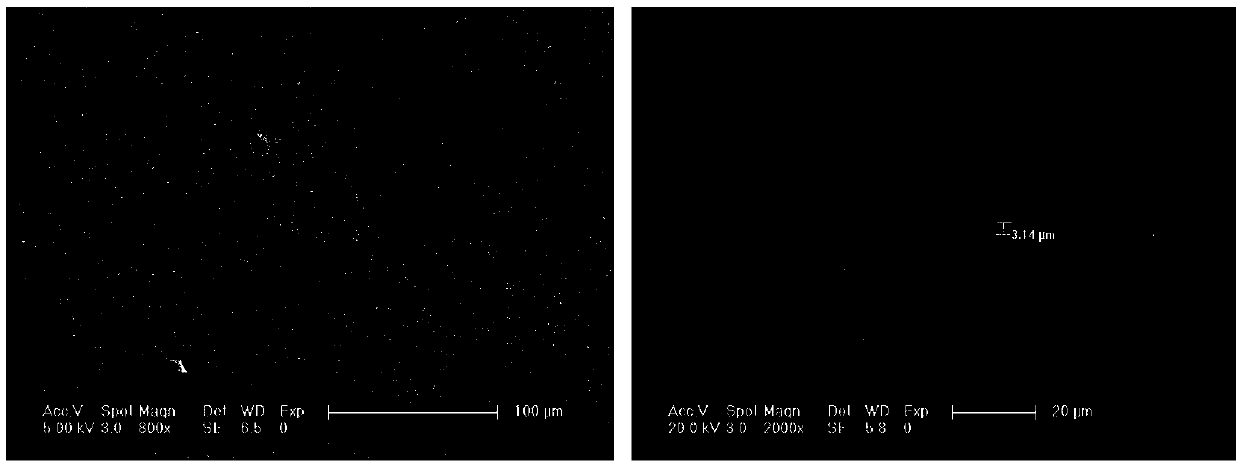
[0097] Weighing Li according to the stoichiometric ratio 1.5 al 0.5 Ge 1.5 (PO 4 ) 3 The raw material Li required for solid electrolyte 2 CO 3 、Al 2 o 3 ,P 2 o 5 、GeO 2 The materials were mixed and dried, and the amorphous block was prepared by melting-quenching method after pre-calcining at 700° C. for 2 hours. The melting temperature is 1400°C, and the holding time is 2h. The amorphous block was pulverized by wet ball milling, pressed at 300MPa and sintered at 900°C for 5h to make a ceramic solid electrolyte sheet.
[0098] Accurately weigh cellulose nitrate 2 times the mass of the amorphous powder and ethanol 8 times the mass, put them together in a beaker and stir for 24 hours under an argon atmosphere to obtain a composite slurry; apply the composite slurry in sequence by scraping in Li 1.5 al 0.5 Ti 1.5 (PO 4 ) 3 Both sides of the solid electrolyte sheet are placed in a muffle furnace after the solvent is volatilized, and heat-treated at 500 ° C for 2 ho...
Embodiment 2
[0104] Will Li 0.35 La 0.55 TiO 3 Electrolyte raw material La 2 o 3 , Li 2 CO 3 and TiO 2 Weigh it with the agate ball according to the mass ratio of 1:30, and place it in an agate jar for dry grinding for 50 hours. During this period, the ball milling tank was opened every 2 hours, the powder sticking to the inner wall of the ball milling tank was scraped off, and then the ball milling was continued to obtain amorphous Li 0.35 La 0.55 TiO 3 Electrolyte powder.
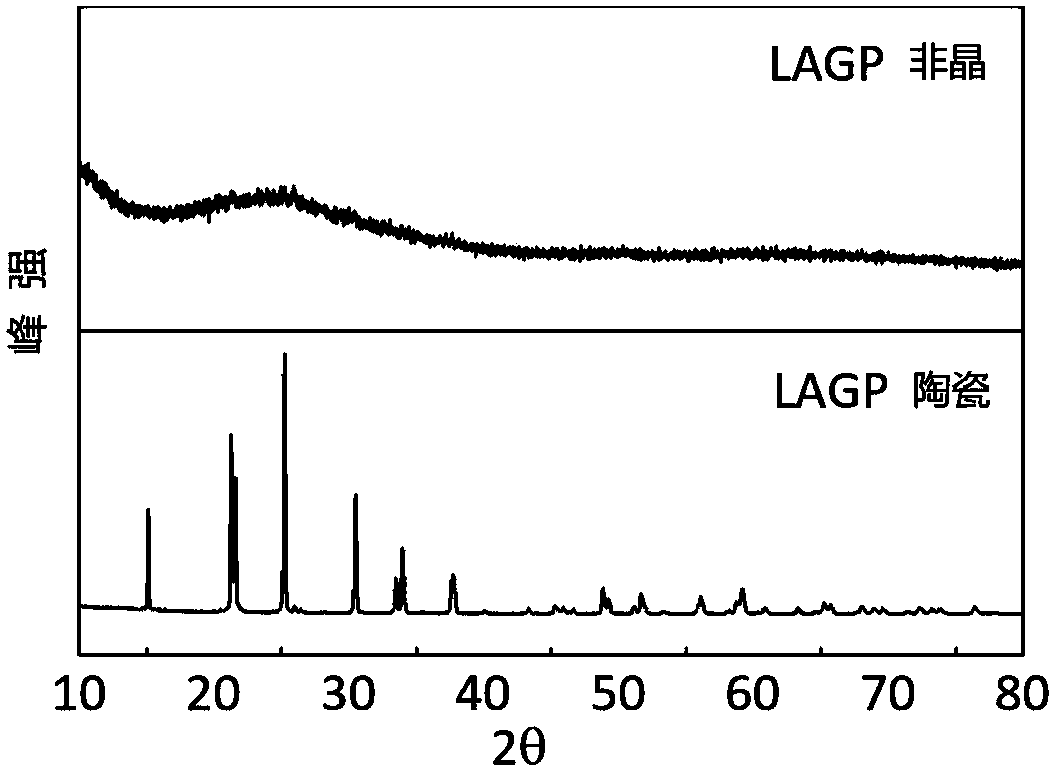
[0105] The obtained powder was pressed into tablets and annealed at 1000°C for 3 hours to obtain Li 0.35 La 0.55 TiO 3 ceramic electrolyte sheets. The resulting amorphous Li 0.35 La 0.55 TiO 3 powder and crystalline Li 0.35 La 0.55 TiO 3 Ceramic sheet XRD comparison see Figure 5 . Figure 5 Amorphous and crystalline Li prepared for Example 2 0.35 La 0.55 TiO 3 The XRD comparison chart.
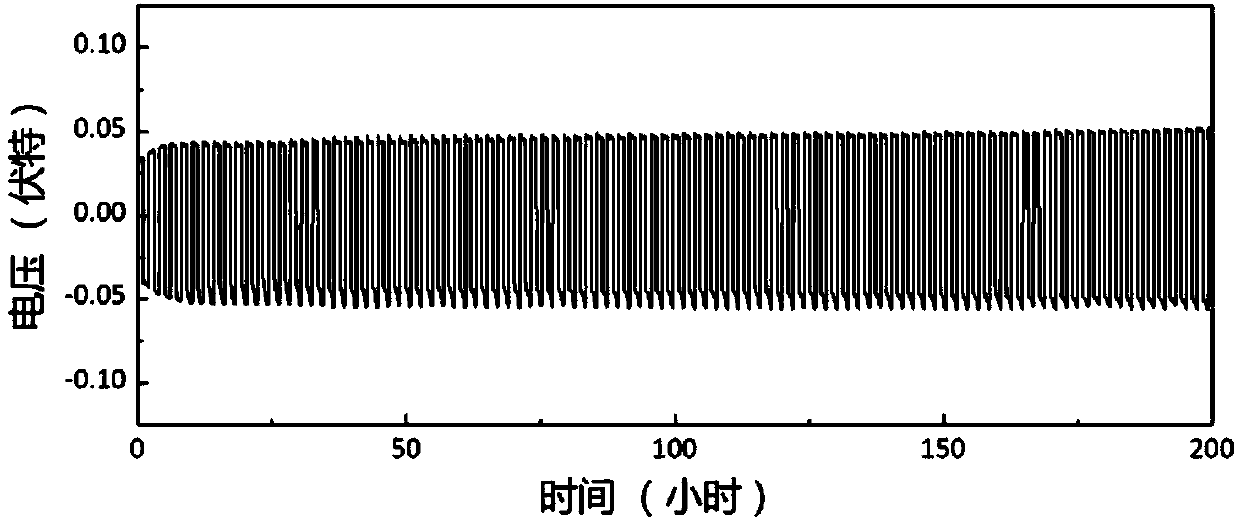
[0106] The amorphous powder, polyvinyl alcohol and dimethyl sulfoxide were weighed according to the mass r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com