Glycopeptide derivative and its pharmaceutically acceptable salts, and their preparation method and application
A derivative and pharmaceutical technology, applied in the field of medicinal chemical synthesis, can solve the problem of reducing the therapeutic effect of compounds and achieve good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, the synthesis of compound LYSC-10
[0061] At room temperature, compound (II) (2.0 g, 1.2 mmol) was dissolved in 15 mL of DMSO, then DIEA (0.4 mL, 2.4 mmol) and N', N'-dimethylaminopropylenediamine (0.18 mL, 1.4 mmol) were added , stirred evenly, and then put into PyBOP (0.73g, 1.4mmol), after the addition was completed, the reaction solution was stirred at room temperature for 1h.
[0062] Add 250mL of acetone to the above reaction solution, stir to precipitate insoluble matter, let stand, filter with suction, wash the filter cake with acetone and dichloromethane successively, and remove the solvent. Purified by reverse-phase polymer filler UniPS25-300, eluted with methanol-water (volume ratio of methanol to water: 1:4) solution containing 0.04% TFA, concentrated and dried the eluate to obtain 0.66 g of white solid.
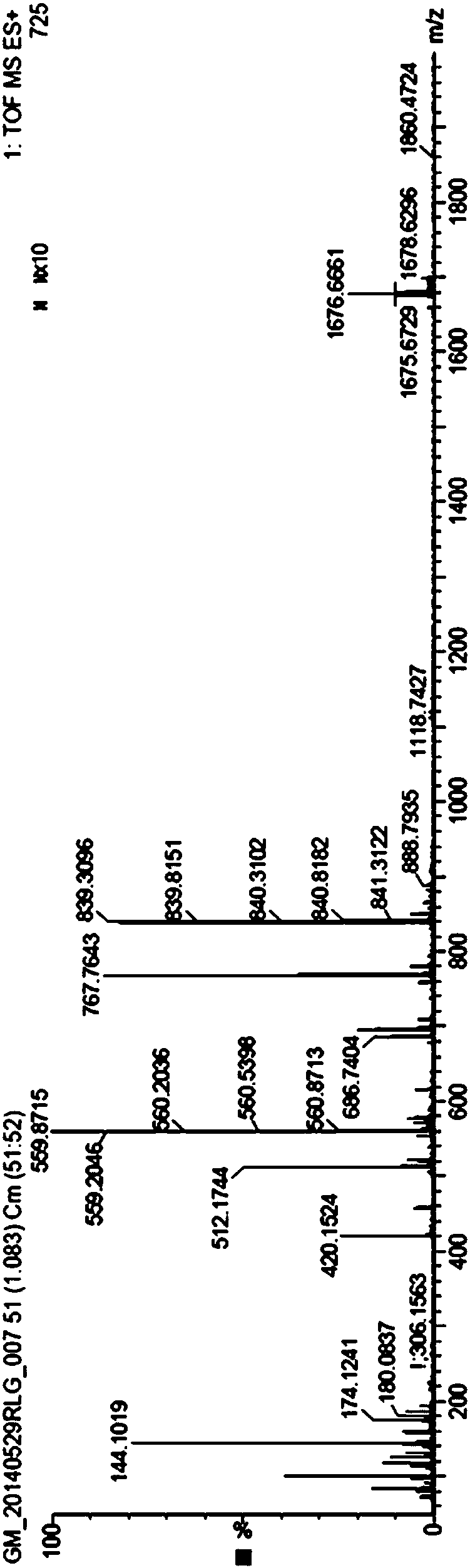
[0063] After testing, the chromatographic purity of the obtained white solid was 97.1%, and the yield was 31.8%. The mass spectrum of the...
Embodiment 2
[0065] Embodiment 2, the synthesis of compound LYSC-14
[0066] At room temperature, compound (II) (0.8g, 0.5mmol) was dissolved in 8mL DMF, then DIEA (0.25mL, 1.5mmol) and 4-cyanobenzylamine (0.1g, 0.75mmol) were added, stirred evenly, and then added TBTU (0.24 g, 0.75 mmol) was added, and the reaction solution was stirred at room temperature for 2 h.
[0067] Add 100mL of acetone to the above reaction solution, stir to precipitate insoluble matter, let stand, filter with suction, wash the filter cake with acetone and dichloromethane successively, and remove the solvent. Purified by reverse-phase polymer filler Uni PS25-300, eluted with methanol-water (volume ratio of methanol to water: 2:3) solution containing 0.03% TFA, concentrated and dried the eluate to obtain 243 mg of white solid.
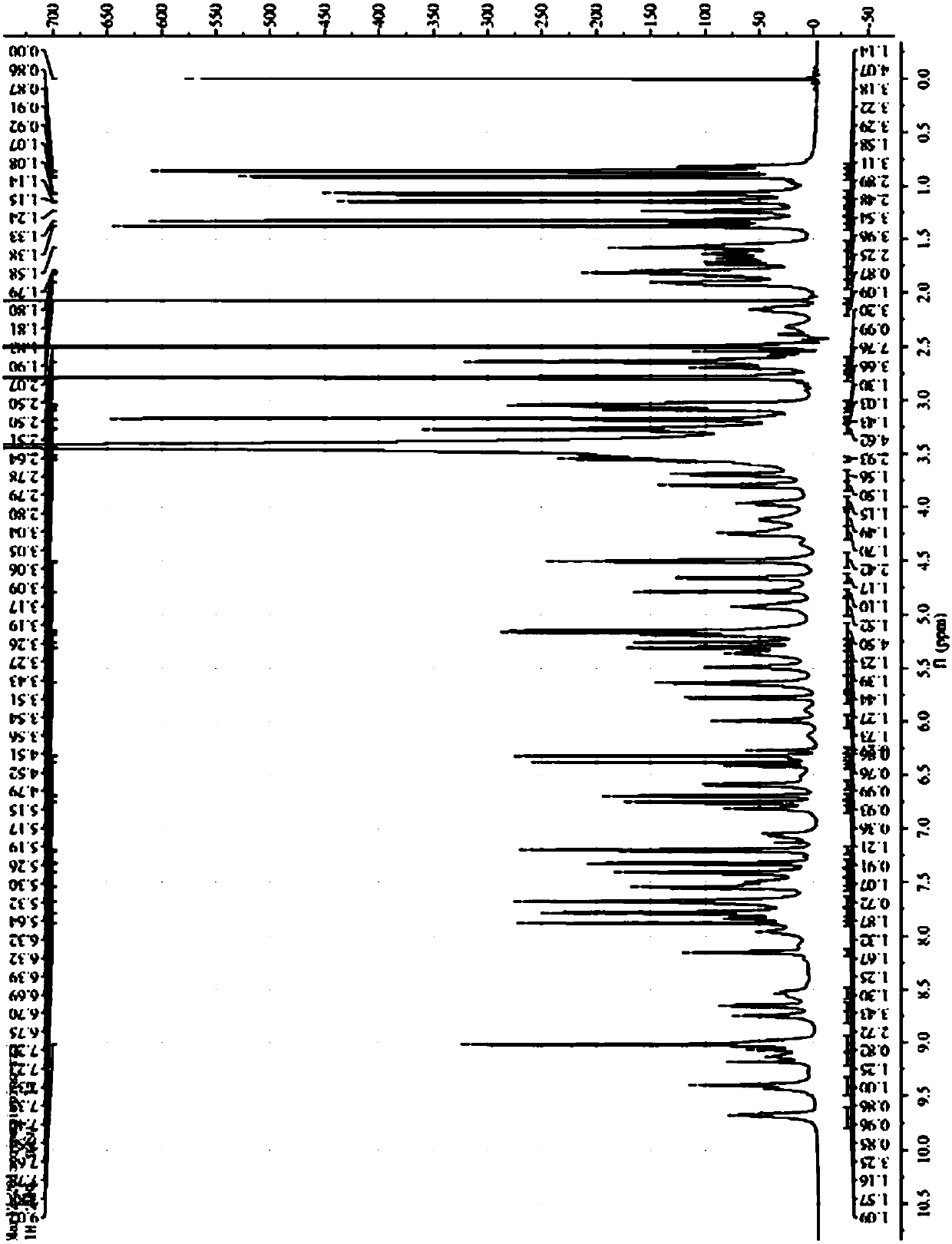
[0068] After testing, the chromatographic purity of the obtained white solid was 96.3%, and the yield was 28.3%. The mass spectrum of the product and 1 H-NMR identification spectrum see ...
Embodiment 3
[0069] Embodiment 3, the synthesis of compound LYSC-38
[0070] At room temperature, compound (II) (500mg, 0.3mmol) was dissolved in 10ml of DMF-methanol (1:1 volume ratio mixing), and then 4'-chlorobiphenyl-4-carbaldehyde (85mg, 0.4mmol) was added, stirred and refluxed for 2h Then add sodium cyanoborohydride (40mg, 0.6mmol), and continue to reflux for 2h. After the reaction solution is cooled, the methanol is evaporated under reduced pressure, and the residue is poured into 50ml of acetone to precipitate insoluble matter. Washing with acetone and dichloromethane and removal of solvent gave a crude solid.
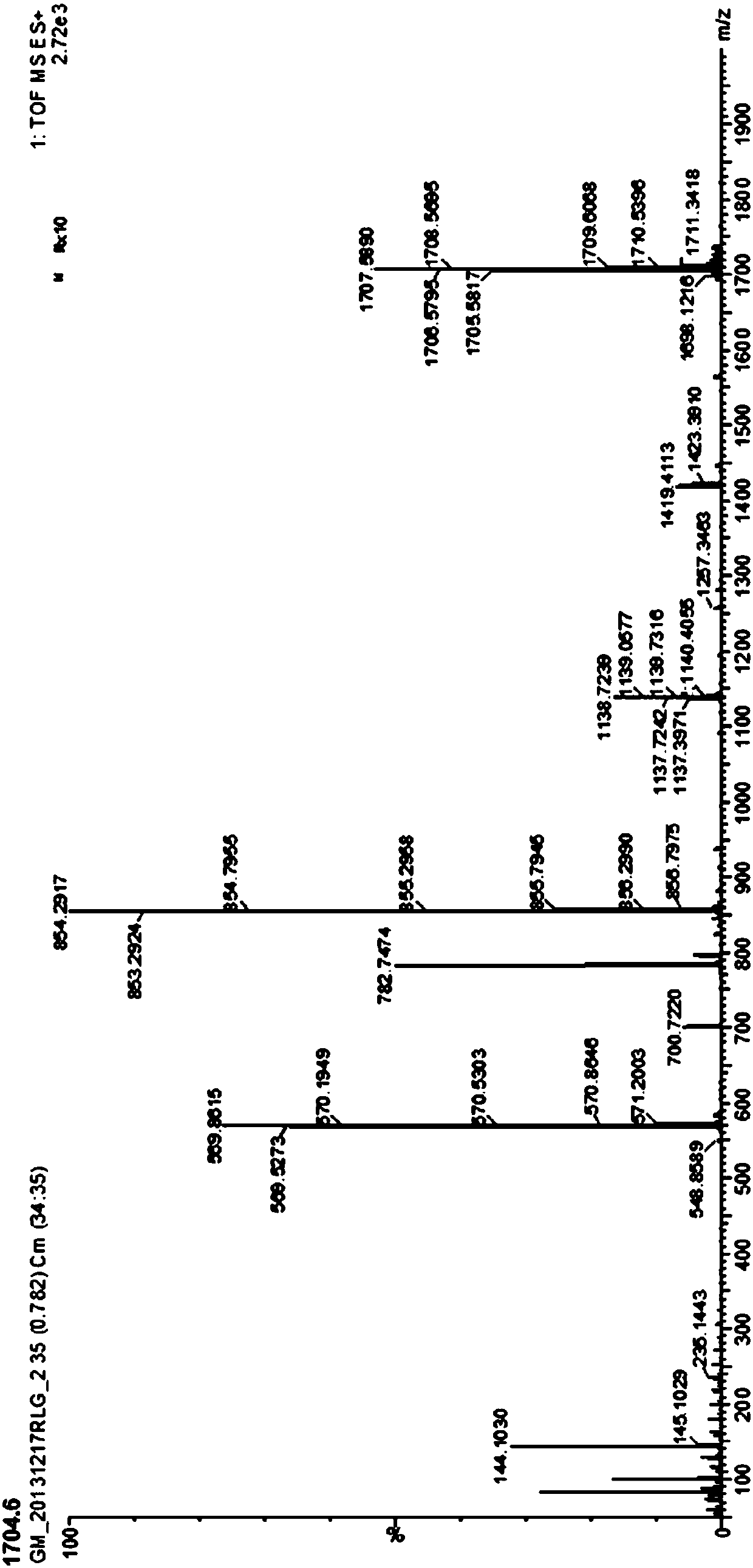
[0071] The resulting crude solid was dissolved in 5 mL of DMSO, DIEA (0.1 mL, 0.6 mmol) and N', N'-dimethylaminopropylenediamine (0.046 mL, 0.36 mmol) were added sequentially, stirred evenly, and then PyBOP (0.2 g, 0.36mmol), the addition was completed, and the reaction solution was stirred at room temperature for 1h. Add 50 mL of acetone to the reaction solution, stir to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com