Icariin nanoparticle and preparation method thereof
An icariin and nanoparticle technology is applied in the field of icariin nanoparticle and its preparation, which can solve the problems of difficult icariin, poor oral absorption, low bioavailability and the like, and achieves simple and cheap materials and high solubility. Improved performance and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
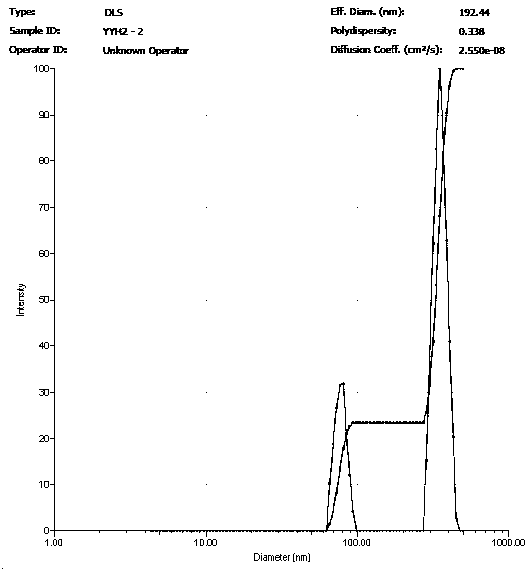
[0022] Dissolve 10 mg of icariin raw material drug in 10 ml of methanol / ethyl acetate solution (volume ratio 1:1) to prepare an icariin solution with a concentration of 1.0 mg / ml; dissolve 300 mg of PVA in 10 ml water, configure a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin solution and add it to 2.25 ml of PVA solution, and then carry out ultrasonic emulsification of the mixture; the ultrasonic frequency is 20-25 KHz, the power is 250 W, the ultrasonic time is 6 min, every ultrasonic 5 s, interval 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000 rpm) to collect the precipitate and add Resuspend in deionized water and centrifuge again. The operation was repeated 5 times, and the nanoparticles were cleaned to obtain ...
Embodiment 2
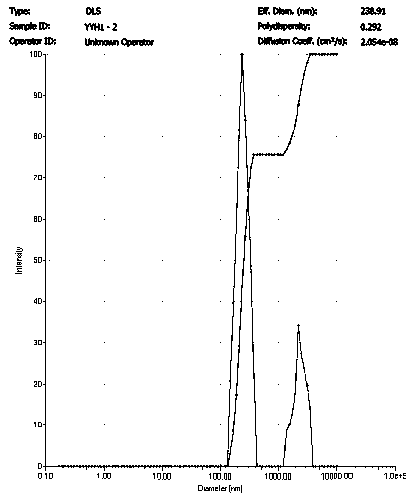
[0025] Dissolve 20 mg of icariin crude drug in 10 ml of methanol / dichloromethane solution (volume ratio of 5:1) to prepare an icariin solution with a concentration of 2.0 mg / ml; dissolve 300 mg of PVA in 10 ml water, configure a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin solution and add it to 2.25 ml of PVA solution, and then carry out ultrasonic emulsification of the mixture; the ultrasonic frequency is 20-25 KHz, the power is 100 W, the ultrasonic time is 6 min, every ultrasonic 5 s, interval 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000 rpm) to collect the precipitate and add Resuspend in deionized water and centrifuge again. The operation was repeated 5 times, and the nanoparticles were cleaned to obtain ic...
Embodiment 3
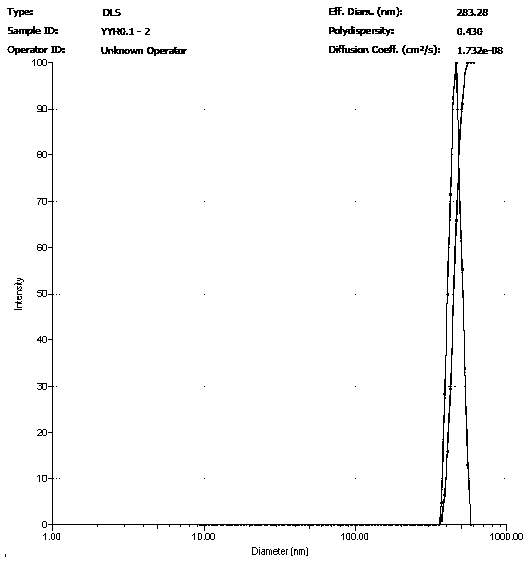
[0027] Dissolve 30 mg of icariin raw material drug in 10 ml of methanol / chloroform solution (volume ratio of 9:1), and prepare an icariin solution with a concentration of 3.0 mg / ml; dissolve 300 mg of PVA in 10 ml of water, configure a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin solution and add it to 2.25 ml of PVA solution, and then carry out ultrasonic emulsification of the mixture; the ultrasonic frequency is 20-25 KHz, the power is 400 W, the ultrasonic time is 6 min, every ultrasonic 5 s, interval 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000rpm), and the precipitate was collected and added to Resuspend in deionized water and centrifuge again. The operation was repeated 5 times, and the nanoparticles were c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com