Use of neogambogic acid or derivatives thereof in preparing of drugs for prevention and/or treatment of related diseases caused by bacteria
A new technology of gambogic acid and derivatives, applied in antibacterial drugs, applications, food science, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
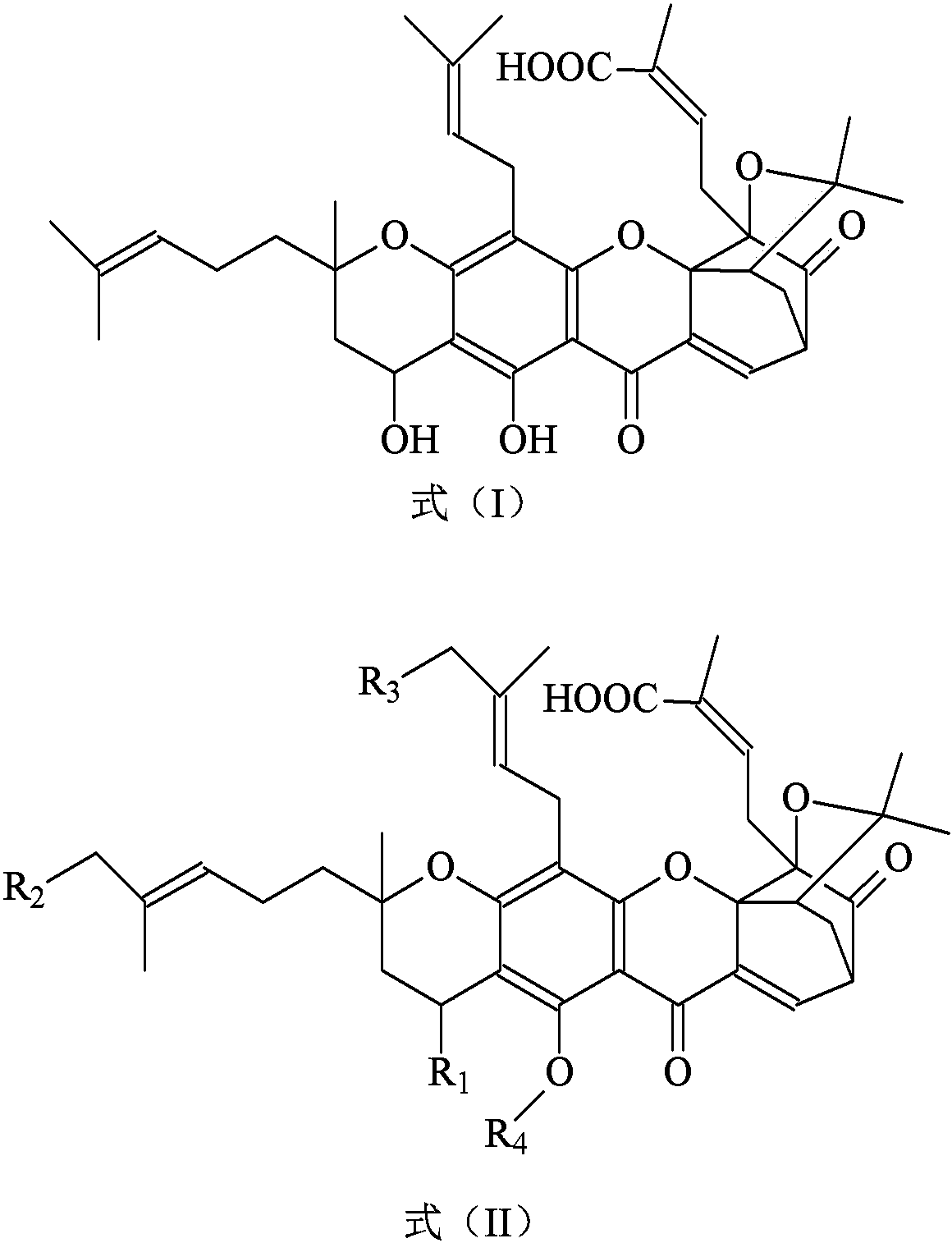
[0024] Embodiment 1 prepares 4-oxo neogambogic acid (XTHS-1)
[0025]
[0026] In a 50mL two-neck flask equipped with mechanical stirring, add acetone (15mL), glacial acetic acid (2mL), neogambogic acid (0.65g, 0.001mol, purchased from Chengdu Pufeide Biotechnology Co., Ltd., article number: JOT10142), After stirring evenly, cool down to about -5°C, add 1.0mL of newly prepared Jones reagent (2.67g of chromium trioxide dissolved in 23ml of concentrated sulfuric acid, and then dilute to 100ml with distilled water) dropwise under stirring, react for 2 hours, add 2mL of ethanol was used to stop the reaction, filtered under reduced pressure, the filtrate was poured into 50mL of ice water, filtered, washed with water, and the filter cake was vacuum-dried to obtain 0.51g of a yellow solid, which was separated on a silica gel column (mobile phase: different mixtures of acetone-ethyl acetate) Volume ratio solution carries out gradient elution), collects the eluted portion of acetone...
Embodiment 2
[0029] Embodiment 2 prepares 35,39-dicyano-neogambogic acid (XTHS-2)
[0030]
[0031] In a 50mL two-necked bottle, add anhydrous pyridine (50mL), neogambogic acid (0.65g, 0.001mol, purchased from Chengdu Pufeide Biotechnology Co., Ltd., article number: JOT10142), N-bromosuccinyl Amine (0.40g, 0.0023mol), after reflux reaction for 12 hours, add sodium cyanide (0.078g, 0.002mol), filter under reduced pressure, pour the filtrate into 500mL ice water, filter, wash with water, the filter cake is vacuum-dried, Obtain yellow solid 0.81g, separate through silica gel column (mobile phase is: the solution of different volume ratios of acetone-ethyl acetate carries out gradient elution), collect the elution part of acetone-ethyl acetate (10:1 volume ratio), subtract After recovering the solvent under pressure, 0.41 g of 35,39-dinitrile neogambogic acid (formula (V)) was obtained, with a yield of 57.40%.
[0032] 1 H-NMR (CDCl 3 , 300MHz) δ: 12.80 (1H, S, COO H), 7.53(1H, d, H-10)...
Embodiment 3
[0034] Example 3 Preparation of 10-cyanohydrogenated neogambogic acid (XTHS-3)
[0035]
[0036] In a 50mL two-necked bottle, add anhydrous pyridine (50mL), neogambogic acid (0.65g, 0.001mol, purchased from Chengdu Pufeide Biotechnology Co., Ltd., article number: JOT10142), hydrocyanic acid (0.027g, 0.001 mol), at about 0°C, after stirring and reacting for 24 hours, the reaction solution was poured into 500mL of ice water, filtered, washed with water, and the filter cake was vacuum-dried to obtain 0.71g of a light yellow solid, which was separated through a silica gel column (mobile phase: Different volume ratio solutions of acetone-ethyl acetate carry out gradient elution), collect the elution part of acetone-ethyl acetate (10:3 volume ratio), after decompression recovery solvent, obtain 10-cyanohydrogenated neogambogic acid ( Formula (VI)) 0.54g, the yield is 80.23%.
[0037] 1 H-NMR (CDCl 3 , 300MHz) δ: 12.80 (IH, S, COO H ), 7.53(1H, d, H-10), 6.45(1H, t, H-27), 5.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com