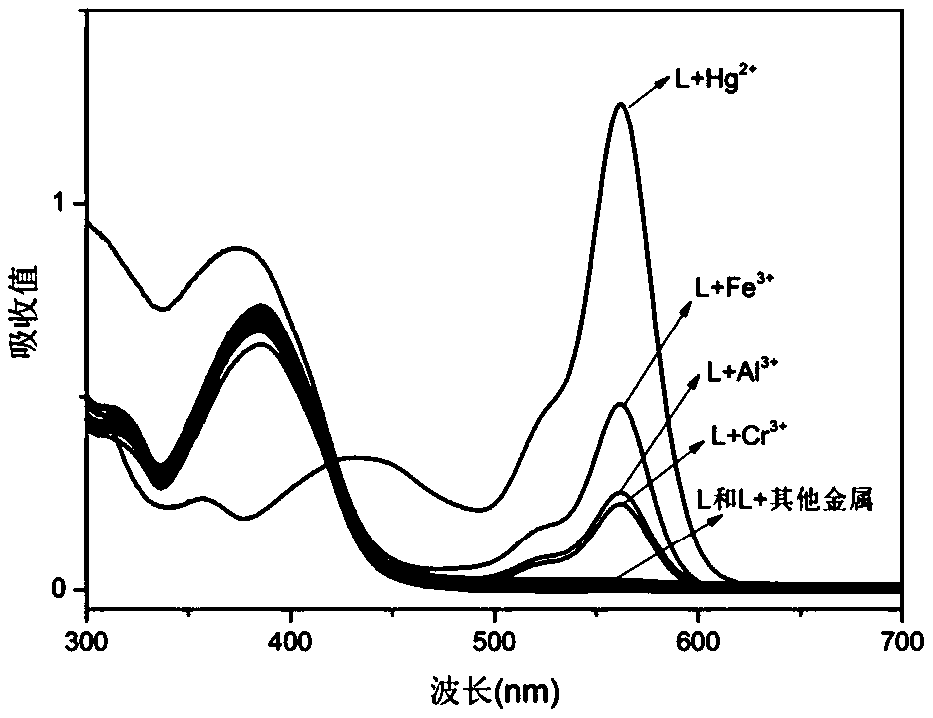
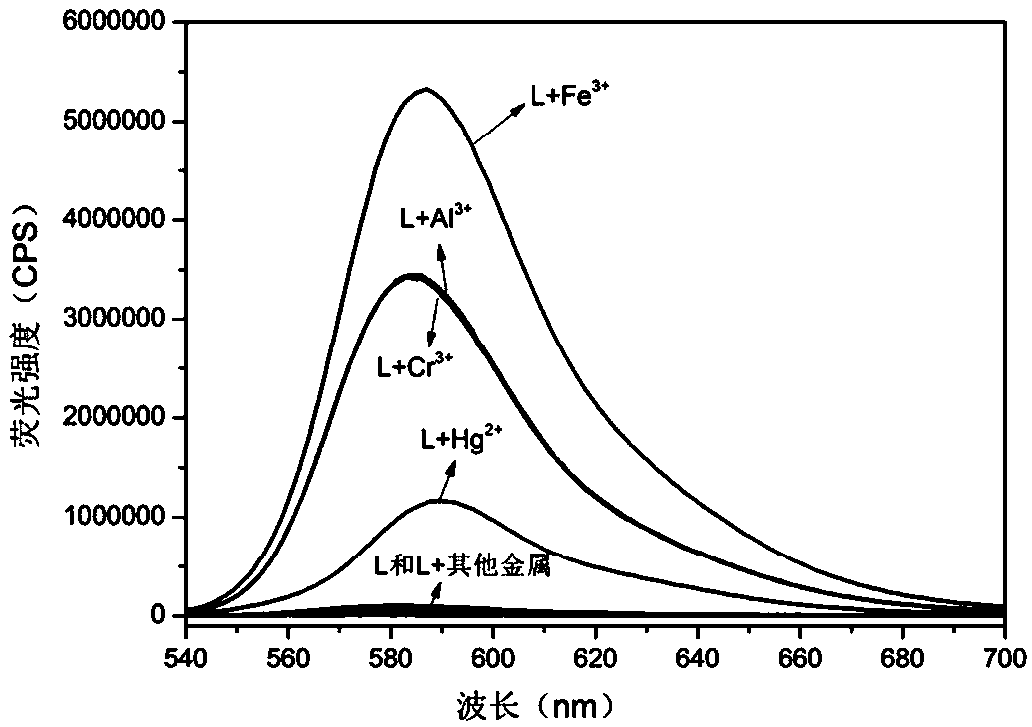
A multi-channel fluorescent probe based on rhodamine b derivatives and its preparation method and application
A fluorescent probe, multi-channel technology, applied in the field of fluorescent probes, can solve problems such as adverse effects on the central nervous system, hematopoietic system, immune system, damage to cell structure and composition, gastrointestinal mucosal damage, etc., and achieve multi-functionality. , strong operability, the effect of saving detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
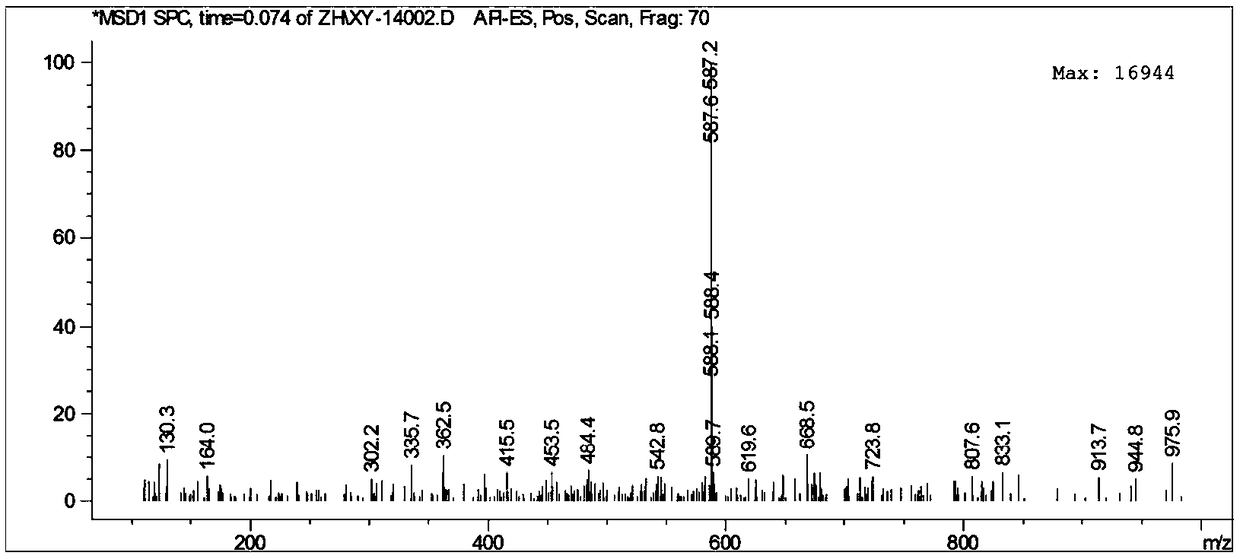
[0027] The reaction formula for synthesizing a multi-channel fluorescent probe based on rhodamine B derivatives is as follows:
[0028]
[0029] Its preparation method comprises the following concrete steps:
[0030] (1) Synthesis of Compound A: Weigh (1.2g, 2.5mmol) rhodamine B, dissolve it in 30mL absolute ethanol, stir vigorously at room temperature, add 5.0mL 85% to 90% hydrazine hydrate dropwise, and dropwise add Then continue to stir for 0.5h, then raise the temperature and reflux for 12h until the solution turns orange-yellow, cool down, remove the solvent by rotary evaporation under reduced pressure, add 1mol / L HCl 50mL, add 1mol / L NaOH 70mL under stirring conditions until the pH of the solution is about 9 , a large amount of precipitation appeared, suction filtered, washed 3 times with distilled water, placed in a vacuum oven and dried overnight to obtain Compound A with a yield of 78%; wherein, the molar ratio of Rhodamine B to hydrazine hydrate was 0.25:1 .
[...
Embodiment 2
[0045] The reaction formula for synthesizing a multi-channel fluorescent probe based on rhodamine B derivatives is as follows:
[0046]
[0047] The preparation method comprises the following steps:
[0048] (1) Synthesis of Compound A: Weigh (1.2g, 2.5mmol) Rhodamine B, dissolve it in 40mL of absolute ethanol, stir vigorously at room temperature, add 2.5mL of 85% to 90% hydrazine hydrate dropwise, after the addition is complete Continue to stir for 0.5h, then raise the temperature and reflux for 12h until the solution turns orange-yellow, cool, remove the solvent by rotary evaporation under reduced pressure, add 1mol / L HCl 50mL, add 1mol / L NaOH 70mL under stirring conditions until the pH of the solution is about 9, A large amount of precipitate appeared, filtered with suction, washed with distilled water for 3 times, and dried overnight in a vacuum oven to obtain Compound A with a yield of 78%; wherein, the molar ratio of rhodamine B to hydrazine hydrate was 0.5:1.
[004...
Embodiment 3
[0061] The reaction formula for synthesizing a multi-channel fluorescent probe based on rhodamine B derivatives is as follows:
[0062]
[0063] The preparation method comprises the following steps:
[0064] (1) Synthesis of Compound A: Weigh (1.2g, 2.5mmol) Rhodamine B, dissolve it in 50mL absolute ethanol, stir vigorously at room temperature, add 0.5mL 85% to 90% hydrazine hydrate dropwise, and dropwise complete Then continue to stir for 0.5h, then raise the temperature and reflux for 12h until the solution turns orange-yellow, cool down, remove the solvent by rotary evaporation under reduced pressure, add 1mol / L HCl 50mL, add 1mol / L NaOH 70mL under stirring conditions until the pH of the solution is about 9 , a large amount of precipitation appeared, suction filtered, washed 3 times with distilled water, placed in a vacuum oven and dried overnight to obtain Compound A with a yield of 82%; wherein, the molar ratio of Rhodamine B to hydrazine hydrate was 1:1 .
[0065] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com