Preparation method of D-phenylalanine
A technology of phenylalanine and phenylalanine deaminase, which is applied in the field of enzyme engineering and can solve the problems of high production cost, complicated preparation process of D-phenylalanine, and difficult availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
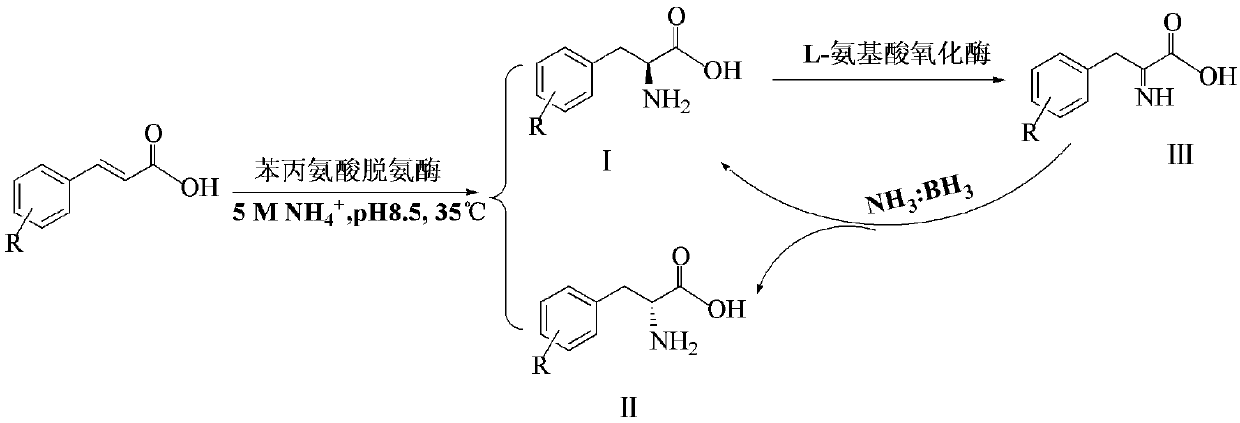
[0023] The present invention provides a method for preparing D-phenylalanine, wherein the preparation method includes: oxidizing trans-cinnamic acid, phenylalanine deaminase, and L-amino acid in the presence of a buffer Enzyme, ammonia water and ammonia borane are mixed to produce high-purity D-phenylalanine.
[0024] Through the above technical solutions, the present invention uses trans-cinnamic acid as the substrate material, which is cheap and has a wide range of sources. Trans-cinnamic acid is catalyzed by phenylalanine deaminase to add ammonia (amination) to L-phenylalanine. Acid and D-phenylalanine. In the reaction system, L-phenylalanine is oxidized by L-amino acid oxidase to generate imine, which is in the reducing agent ammonia borane (NH 3 BH 3 ) Is reduced to L-phenylalanine and D-phenylalanine under the existing conditions, so that the L-phenylalanine produced in the reaction process is continuously converted into D-phenylalanine. Through the continuous cycle convers...
preparation example
[0033] The gene sequence of phenylalanine deaminase in Anabaena variabilis (for details, see the sequence published by NCBI with the accession number LF643444.1) and the gene sequence of L-amino acid oxidase in Proteus mirabilis (for details, see the accession number published by NCBI) For EU669819.1) submitted to Shanghai Shenggong Bioengineering Technology Co., Ltd. for synthesis, the gene for phenylalanine deaminase was named pal, and the gene for L-amino acid oxidase was named laao. The two genes pal and laao were respectively Connect with the cloning vector pUC vector to obtain cloning vectors pUC-pal and pUC-laao.
[0034] The pUC-laao and empty expression vector pET28a were double digested with restriction enzymes EcoRI and NdeI, respectively. pUC-pal and empty expression vector pET28a were double digested with BamH I and Not I, respectively, and the target gene fragment and pET28a vector, then ligate the double digestion product overnight at 16°C, transfer the ligation pr...
Embodiment 1
[0038] Take 50mg of the mixture of phenylalanine deaminase and L-amino acid oxidase prepared above and add it to the substrate trans-cinnamic acid containing 10mmol / L, 5mol / L ammonia (NH 3 H 2 O), 40mmol / L NH 3 BH 3 In the Tris hydrochloric acid buffer solution (pH8.5), the reaction was carried out at 35°C for 16 hours. The content of D-phenylalanine and L-phenylalanine was determined by HPLC, and the conversion rate and D of the substrate trans-cinnamic acid were calculated. -Optical purity of phenylalanine (ee D ). Where ee D Calculate according to the following formula:
[0039]
[0040] HPLC determination results such as Figure 4 Shown.
[0041] It can be seen from the figure that after the conversion, L-phenylalanine is not detected, so the optical purity of D-phenylalanine (ee D ) Over 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com