Separation and determination method of erlotinib hydrochloride and potential impurities
An erlotinib hydrochloride, a potential technology, applied in the direction of material separation, measuring device, analysis material, etc., can solve the problem of not simultaneously separating and measuring erlotinib hydrochloride, etc., achieve high sensitivity, ensure product quality, and strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
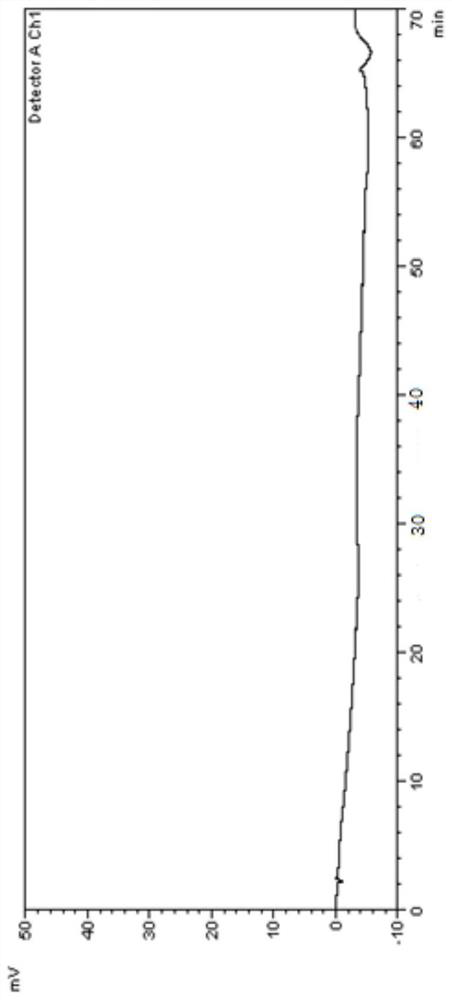
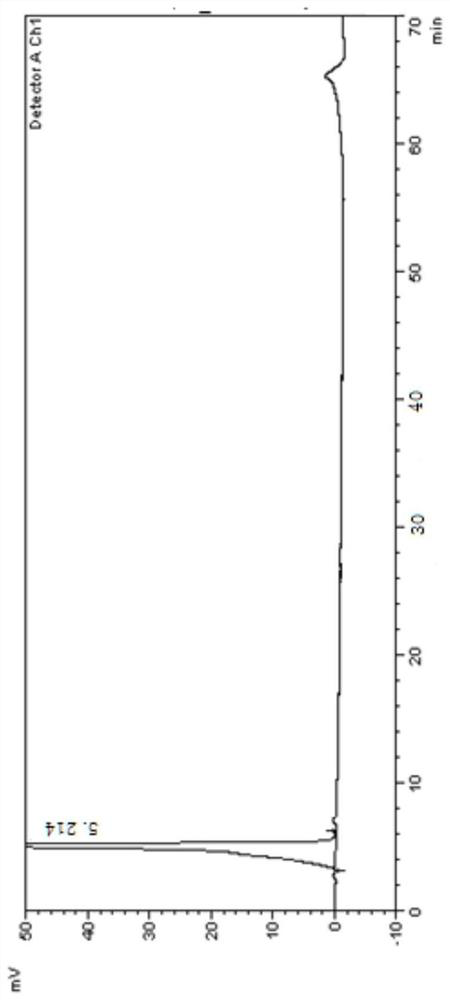
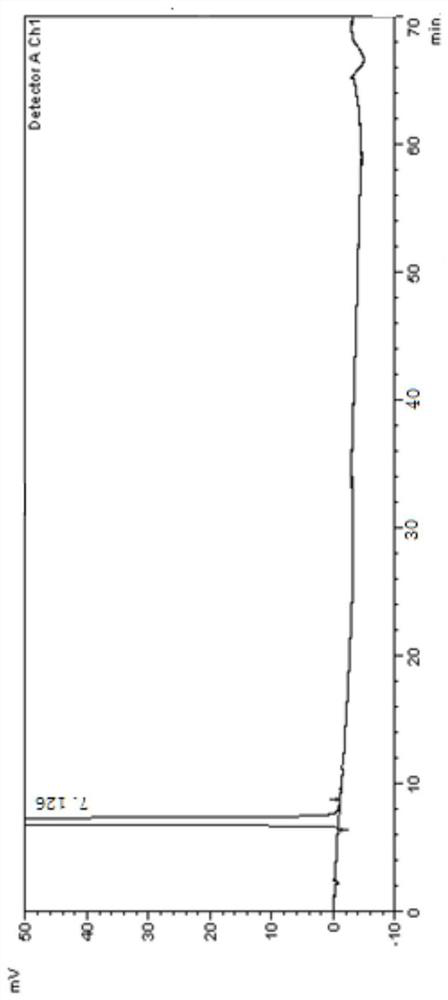
[0099] 1. Instruments and conditions
[0100] Instrument: high performance liquid chromatography;
[0101] Chromatographic column: Agilent Eclipse XDB C18 (4.6×250mm, 5μm);
[0102] Mobile phase: Carry out linear gradient elution as shown in Table 1;
[0103] A: Phosphate buffer salt (take 1.36g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, add 2.0ml of diethylamine solution, mix well, adjust the pH value to 3.1±0.1 with phosphoric acid solution);
[0104] B: methanol-acetonitrile (volume ratio 1:1);
[0105] Table 1 Gradient elution table
[0106]
[0107] UV detector detection wavelength: 247nm;
[0108] Flow rate: 1.0ml / min;
[0109] Column temperature: 30°C;
[0110] Injection volume: 20μl;
[0111] Diluent: acetonitrile aqueous solution (volume ratio 1:1).
[0112] 2. Experimental steps
[0113] (1) Positioning solution of each impurity: Accurately weigh the appropriate amount of each impurity reference substance, put it in dif...
Embodiment 2
[0120] Embodiment 2 The method of the present invention and the comparison of existing analytical method
[0121] Erlotinib hydrochloride is not recorded in Ch.P, USP and EP, so refer to the related substance analysis method in the National Imported Drug Registration Standard JX20080202 of Erlotinib Hydrochloride Tablets (see Table 2 methods 1-3), but this The method does not control for known impurities.
[0122] Method 4 in Table 2 is the method recorded in the reference "Qualitative and Quantitative Analysis of 5 Related Substances in Erlotinib Hydrochloride and Its Tablets" [from "Journal of Pharmaceutical Analysis", Issue 5, 2015].
[0123] Method 5 in Table 2 is the method of the present invention.
[0124] Table 2 Analysis methods of related substances
[0125]
[0126]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com