Antiviral compound and its preparation method and application
An antiviral and compound technology, applied in the field of new compounds with antiviral activity and their preparation, can solve the problems of strong toxicity and side effects, expensive drugs, etc., and achieve strong antiviral activity, simple separation and purification, and good dose dependence Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
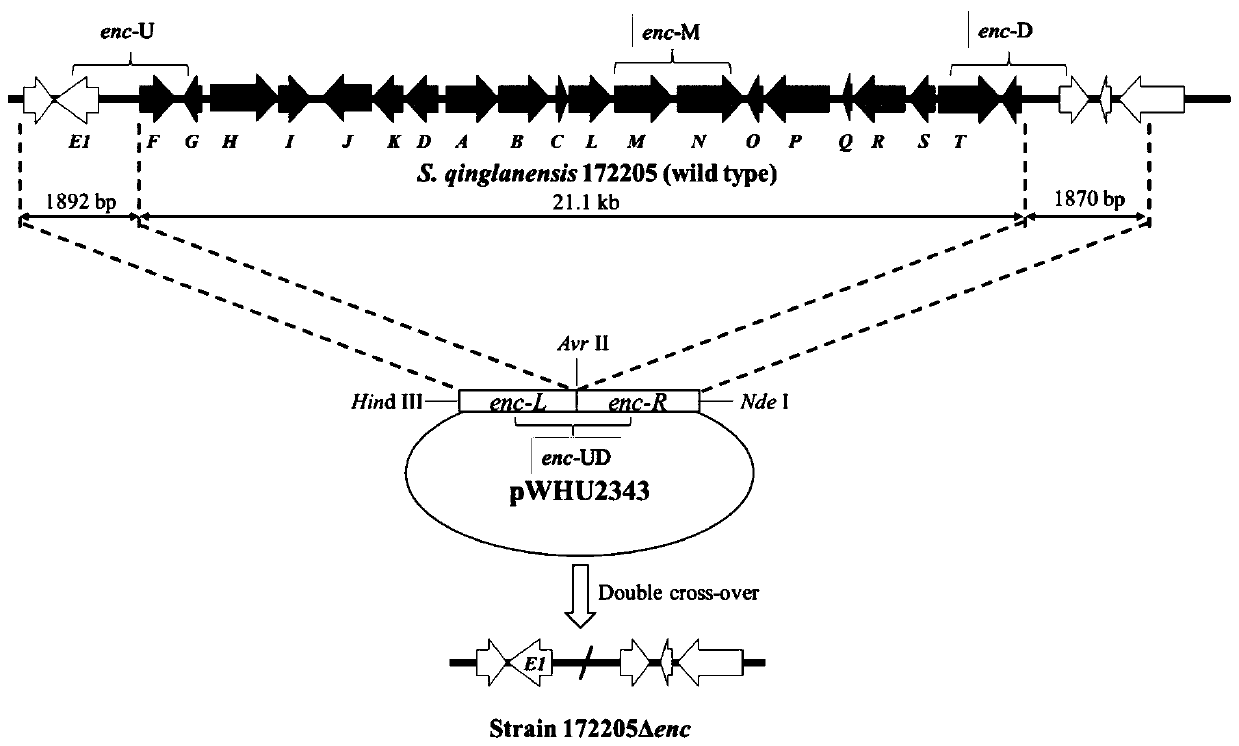
[0040] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] Construction of enterocin biosynthetic gene cluster deletion mutant strain Δenc
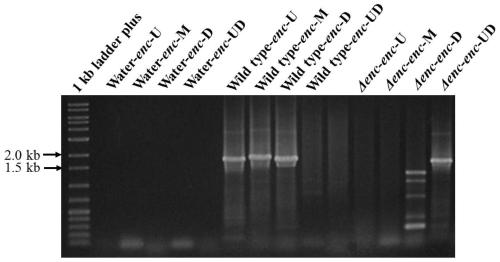
[0041] Using the genomic DNA of wild-type 172205 as a template, primers enc-L1 (5'-CCTAGGCAGTTCCATCACCCCGTTCG-3', SEQ NO.1) and enc-L2 (5'-AAGCTTGTCGTCGCAGCAGCAGTTCG-3', SEQ NO.2) were designed for amplification To increase the upstream fragment enc-L of the enterocin synthetic gene cluster, design primers enc-R1 (5'-CATATGAGAGGGCGGACGGGAACTGC-3', SEQ NO.3) and enc-R2 (5'-CCTAGGGCGCCATCCCAACGGGCTAC-3', SEQ NO.4) for Amplify the downstream fragment enc-R of enterocin synthesis gene cluster. 20 μL PCR reaction system using Taq Mix enzyme: 8 μL Mix enzyme, 1 μL each pr...
Embodiment 2
[0045] [Example 2] Large-scale fermentation of the mutant strain 172205Δenc and its pretreatment method for fermented product samples
[0046] The mutant strain 172205Δenc obtained in Example 1 was inoculated in ISP2 liquid medium, cultured at 28°C and 200r / min for 3 days, and transferred to 12 shake flasks containing 100mL fermentation medium at 28°C with a 5% inoculum size. , 200r / min to continue culturing for 7 days to obtain a fermentation broth. The fermentation broth was directly extracted three times with an equal volume of ethyl acetate, during which ultrasonic waves and vibrations were performed several times, the ethyl acetate layers were combined, and concentrated to dryness under reduced pressure at 35°C to obtain a crude extract. The ISP2 liquid medium is composed of the following components: glucose 4g / L, yeast extract 4g / L, malt extract 10g / L, and the pH value is 7.2. The fermentation medium is composed of the following components: glucose 20g, soluble starch 1...
Embodiment 3
[0047] [Example 3] Isolation and structure confirmation of compounds
[0048] (1) Compound separation
[0049] About 1 g of the crude extract obtained in Example 2 was dissolved in an appropriate amount of methanol and then mixed into reverse silica gel, and the dry loading was carried out by reverse silica gel chromatography (methanol / water elution system) for elution and separation in sequence. The eluate of the target component was concentrated and then separated and purified using acetonitrile and water with a mobile phase ratio of 30:70 (volume ratio) using C18 semi-preparative liquid phase to obtain the target compound (4 mg).
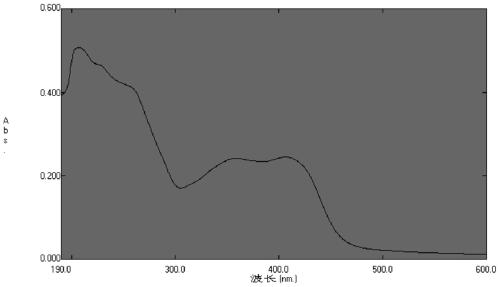
[0050] (2) Planar structure confirmation
[0051] Via UV( image 3 ), IR ( Figure 4 ), MS( Figure 5 ) and NMR ( Figure 6-10 ) a variety of spectroscopic means to determine the structure of the above compound, its physical and chemical properties and spectral data are as follows:
[0052] Light brown powder; optical rotation: Molecular ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com