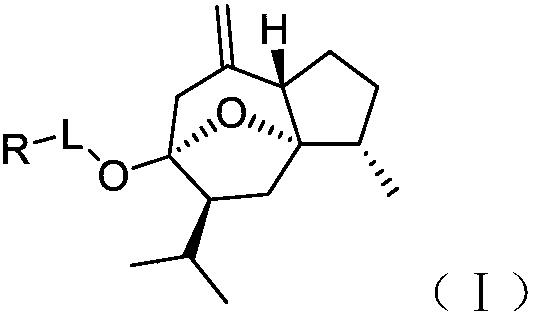
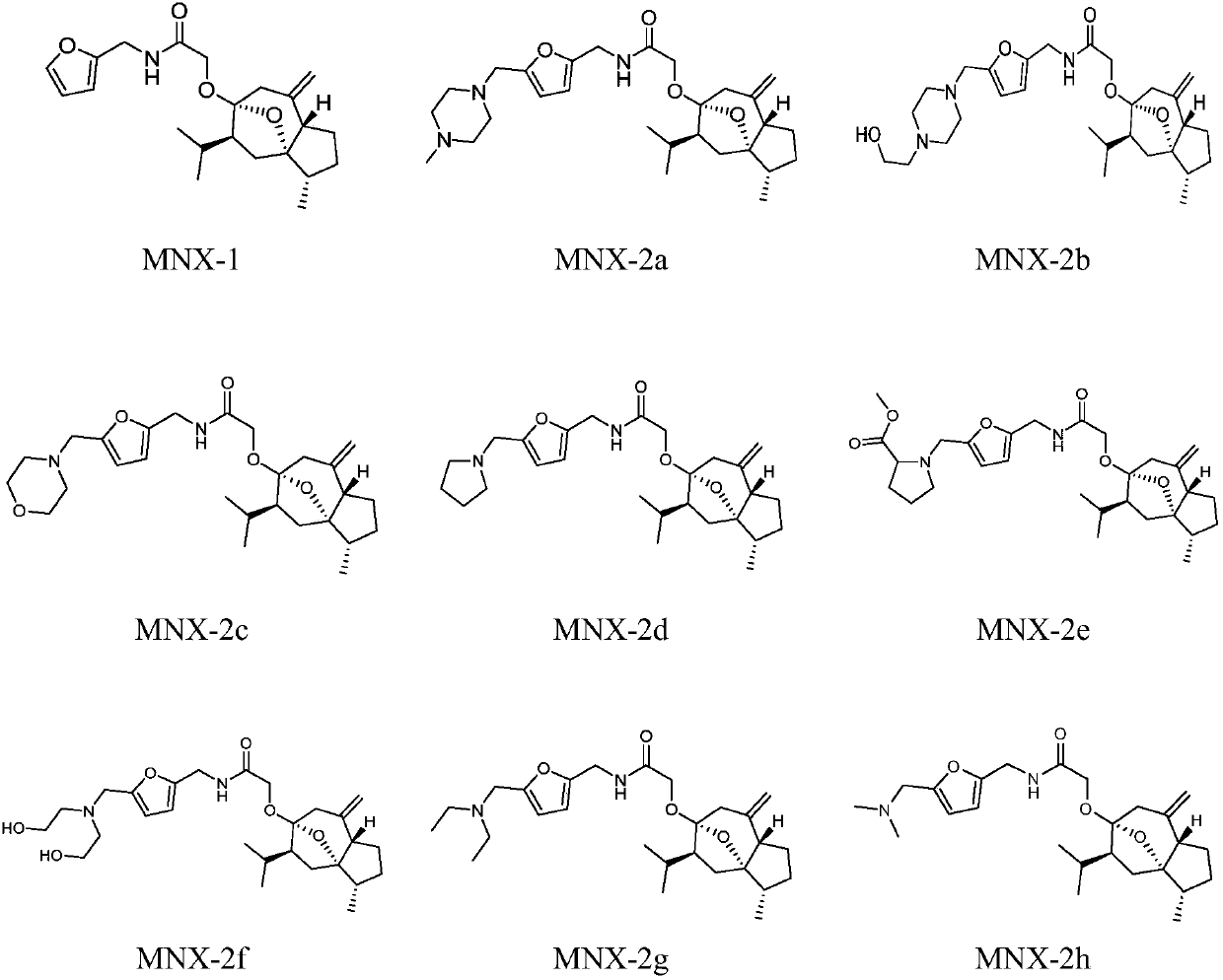
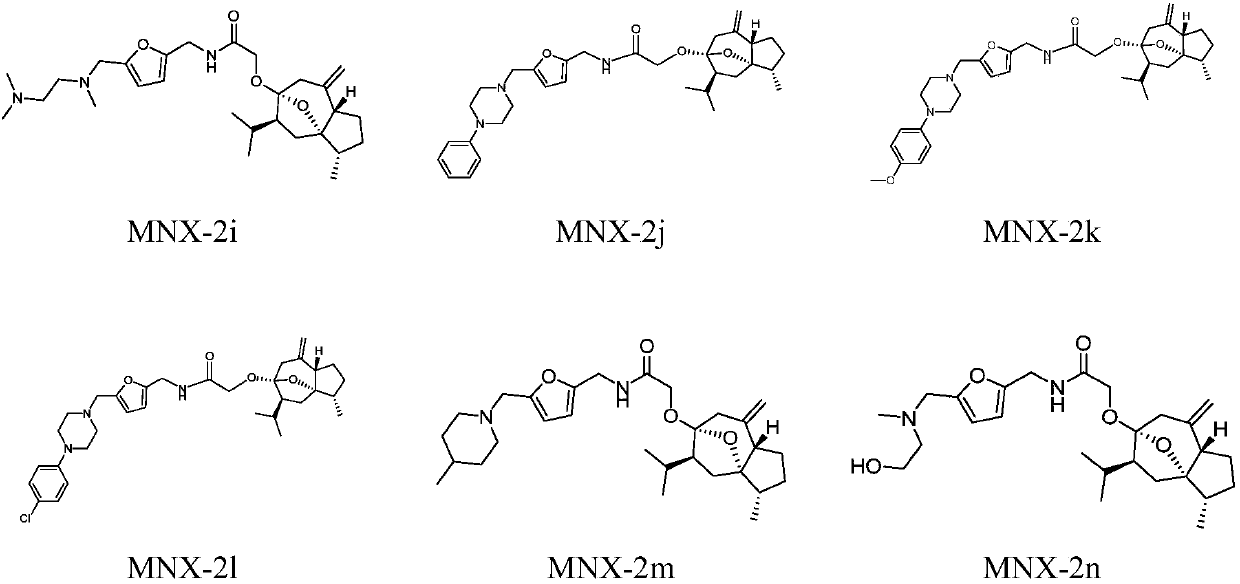
Curcumenol derivatives and application of curcumenol derivatives in preparing antitumor drug
A technology of curcumol and its derivatives, applied in the field of medicine, can solve the problems of clinical application limitations, poor water solubility of curcumol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 curcumol derivative
[0034] The preparation scheme is as follows:
[0035]
[0036] 1. Furfurylamine (25mmol), put it in an eggplant-shaped bottle, add dry tetrahydrofuran to it, add triethylamine (75mmol) as an acid-binding agent, place it on a magnetic stirrer, and slowly add chlorinated acetyl chloride dropwise to it at room temperature (37.5mmol) of tetrahydrofuran solution with constant stirring, 1h dropwise addition is completed, and the reaction is continued at room temperature. The ester layer was backwashed with saturated aqueous sodium chloride solution, dried with an appropriate amount of anhydrous magnesium sulfate, filtered after 1 h, concentrated under reduced pressure to obtain a tan crude product, and dried in a vacuum oven to obtain compound 1, which was directly put into the next reaction.
[0037] 2. Add curcumol (5mmol) into an eggplant-shaped bottle, add dry tetrahydrofuran and stir to dissolve, weigh sodium hydr...
Embodiment 2
[0044] Embodiment 2: in vitro antitumor activity experiment
[0045] Select part of the curcumol derivatives synthesized in Example 1 to carry out in vitro anti-tumor activity experiments, respectively select HepG2 (human liver cancer cells), Hela (human cervical cancer cells), A549 (human lung cancer cells) cell lines, and use MTT (3- (4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide) reduction method was used to determine the inhibitory activity of curcumol-3-substituted ether derivatives on three human cancer cell lines, And calculate the drug concentration when the inhibition rate reaches 50%, ie the IC50 value, and use 5-fluorouracil as the positive control.
[0046] Tumor cells in the logarithmic growth phase were selected, digested with trypsin, and mixed with RPMI 1640 medium to make 6×10 4 / mL of cell suspension, and then add the cell suspension to 96-well culture plate, 37°C, 5% CO 2 Conditioned for 24 hours. Add the pre-configured drugs with different conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com