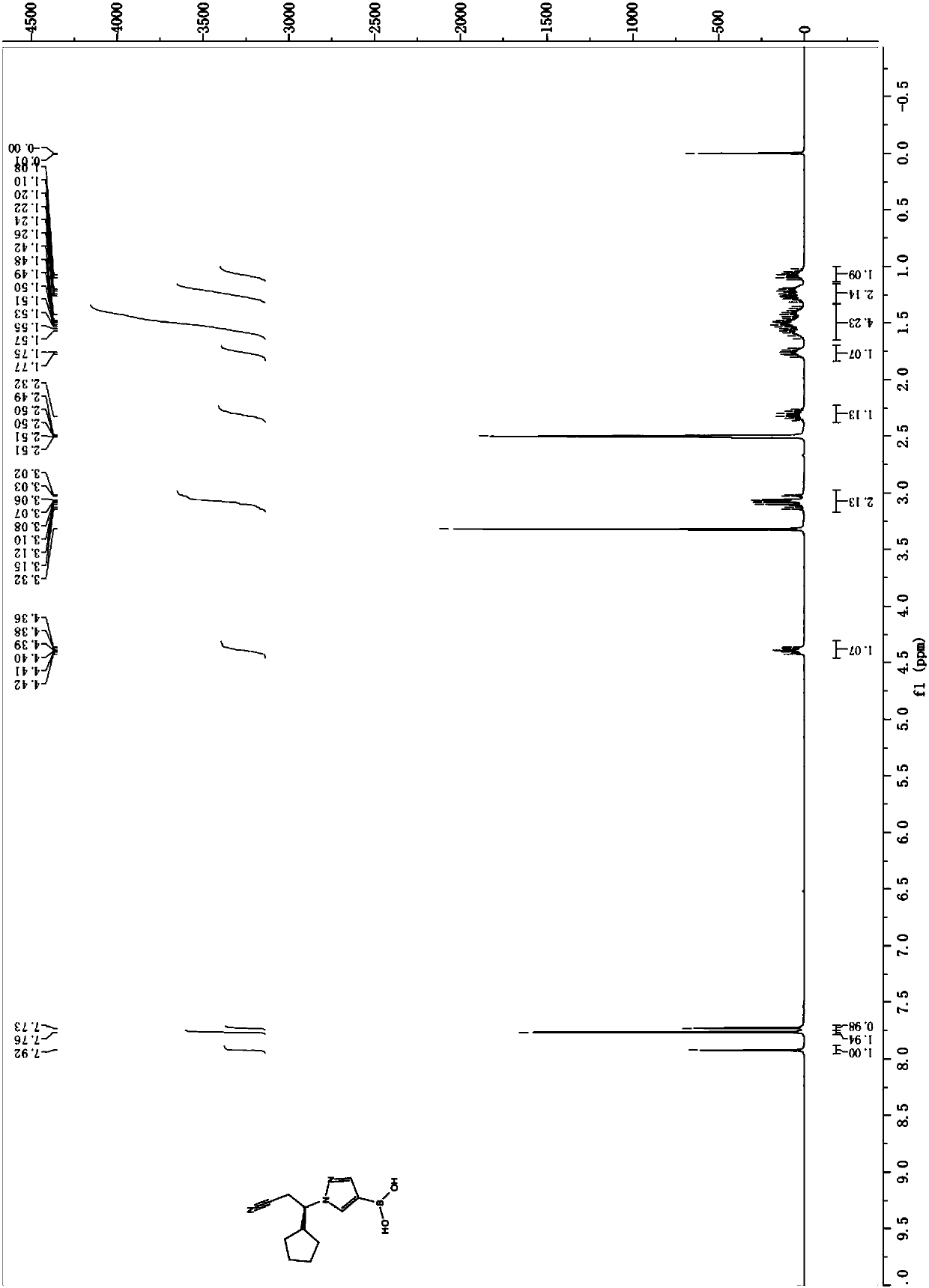
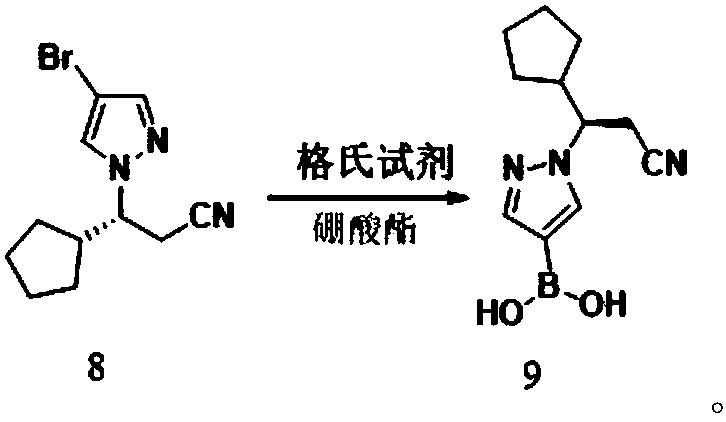
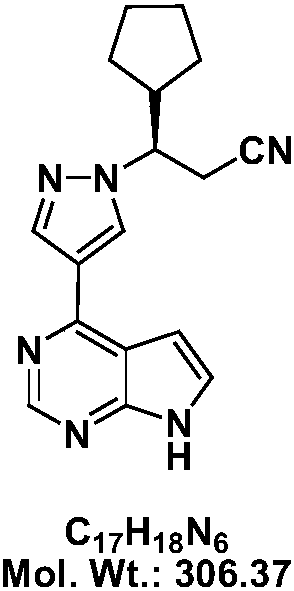
Preparation methods of JAK inhibitor and salt thereof
A solvent and methoxyethylene technology, which is applied in the field of preparation of JAK inhibitor ruxolitinib, can solve problems such as low chiral purity, unsuitable for scale-up production, and difficult purification of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the preparation of cyclopentylcarbaldehyde (compound 2)
[0076] Weigh some bromocyclopentane, dissolve a small amount in 5 times the volume of anhydrous THF, add magnesium chips (1eq), catalytic amount of iodine element, nitrogen protection, raise the temperature to about 40°C to initiate the reaction, control the temperature at 30-40°C and drop Add bromocyclopentane, drop it, keep stirring for 1-2 hours, add DMF (1.05eq) dropwise under temperature control below 10°C, after dropping, stir at room temperature 30°C for 1 hour, add 3 times the volume of MTBE to the feed solution, Add 3N dilute hydrochloric acid dropwise below 0°C to adjust the pH to 3-4, separate the liquids, extract the aqueous layer twice with MTBE (3 times the volume each time), until the product is basically extracted, combine the organic phases, wash twice with saturated brine, and anhydrous Na 2 SO 4 Dry, filter, and concentrate to dryness under reduced pressure to obtain crude cyclo...
Embodiment 2
[0077] Embodiment 2: the preparation of methyl 3-cyclopentyl acrylate (compound 3)
[0078] Weigh potassium tert-butoxide (1.05eq), dissolve it in 10 times the volume of anhydrous THF, protect it under nitrogen, and add trimethyl phosphoacetate (1.1eq) dropwise to the feed solution at about 0°C. Stir at around 0°C for 2 to 3 hours, weigh some cyclopentyl formaldehyde, and make 2 times the volume of anhydrous THF solution, add it dropwise to the above feed solution at around 0°C, after the drop is completed, warm it up to room temperature at about 30°C and stir After 12-15 hours of reaction, the reaction is complete. Add 5 times the volume of MTBE to the feed solution for dilution, 10 times the volume of water, and separate the liquids. The water layer is back-extracted twice with 3 times the volume of MTBE, washed twice with saturated brine, and anhydrous Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to obtain crude methyl 3-cyclopentyl acrylate with a yield ...
Embodiment 3
[0079] Example 3: Preparation of methyl 3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentapropionate (compound 4)
[0080] Weigh some methyl 3-cyclopentyl acrylate, dissolve in 5 times the volume of acetonitrile, add 4-bromopyrazole (1.1eq) and DBU (1.5eq) to the feed solution, and stir the feed solution at room temperature at 30°C to react About 12 to 16 hours, the reaction of raw materials is complete. Concentrate under reduced pressure to a small volume, add 5 times the volume of MTBE to the feed liquid to dilute, wash with water, adjust the pH to 3-4 with 1N dilute hydrochloric acid, wash with water, wash with saturated brine, and wash with anhydrous Na 2 SO 4Dry, filter, and concentrate under reduced pressure to obtain crude methyl 3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentapropionate with a yield of 90-95%, which is directly used for the next step of hydrolysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com