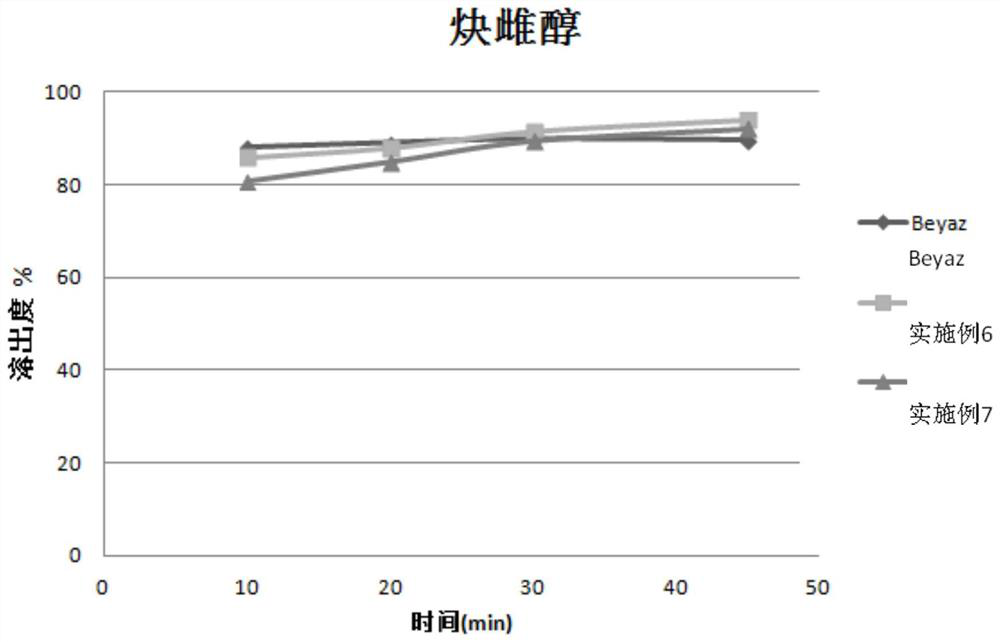
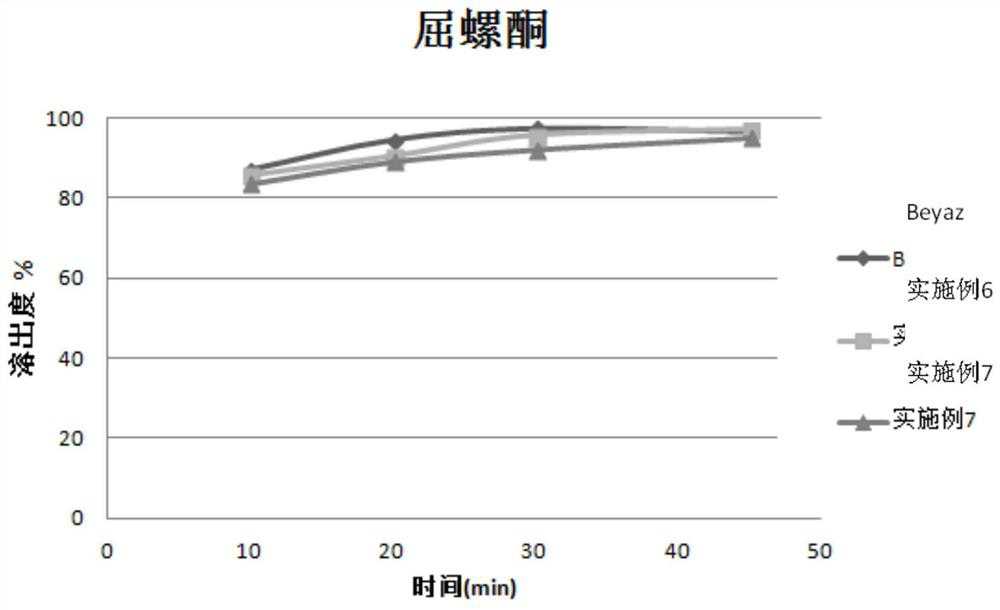
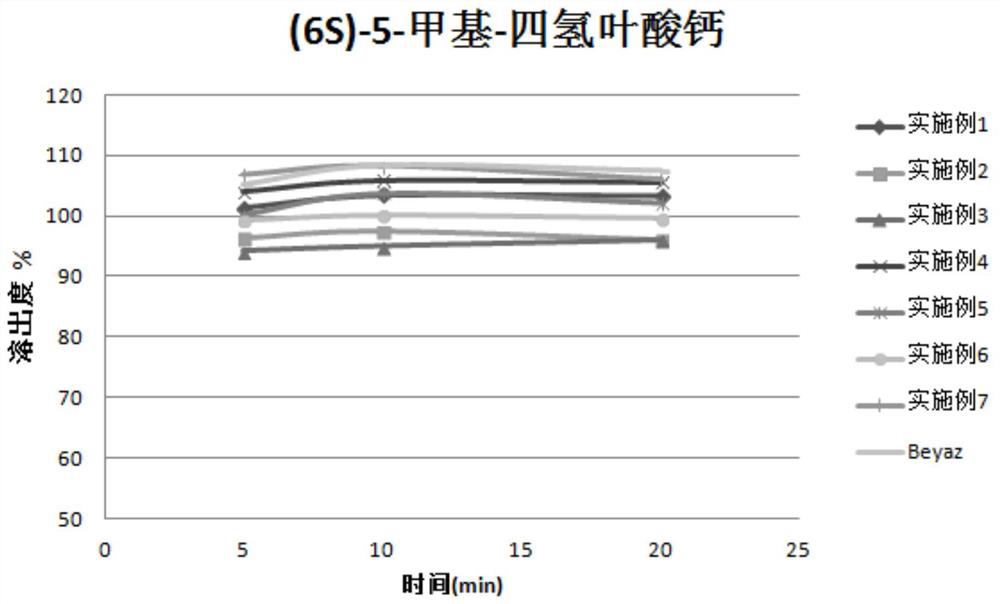
Stable pharmaceutical composition of (6s)‐5‐methyl‐tetrahydrofolate calcium salt
By adding reducing substances such as vitamin C to the drug, 5-methyltetrahydrofolate calcium is protected from oxidation, solving its stability and safety problems, and achieving higher drug stability and lower JK12A content. Reduced risk of venous thrombosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 - direct compression tablet:
[0089] The composition of the prescription is as follows: 80mg / tablet
[0090]
[0091] The preparation method is as follows:
[0092] 1. Pass (6S)‐5‐methyl‐tetrahydrofolate calcium, lactose T80, microcrystalline cellulose PH112, hypromellose, and croscarmellose sodium through a 60-mesh sieve; stearic acid Magnesium through 80 mesh sieve;
[0093] 2. Mix the above-mentioned raw and auxiliary materials except magnesium stearate by doubling dilution method, so that the mixing is uniform;
[0094] 3. Add the prescribed amount of magnesium stearate to the mixture obtained in the above 2 to make the mixture uniform and avoid excessive lubrication.
[0095] 4. The theoretical tablet weight is 80 mg / tablet, and the tablet weight difference is controlled to be no more than 10%, the disintegration time limit is less than 15 minutes, and the friability is less than 1.0%.
[0096] 5.15% Opadry solution is used for film coating, the t...
Embodiment 2
[0097] Example 2 - Direct Compression:
[0098] The composition of the prescription is as follows: 80mg / tablet
[0099]
[0100] Preparation:
[0101] 1. Pass (6S)‐5‐methyl‐tetrahydrofolate calcium, vitamin C, pregelatinized starch PC‐10, microcrystalline cellulose PH112, and carboxymethyl starch sodium through a 60-mesh sieve; magnesium stearate 80 mesh sieve;
[0102] 2. Mix the above-mentioned raw and auxiliary materials except magnesium stearate by doubling dilution method, so that the mixing is uniform;
[0103] 3. Add the prescribed amount of magnesium stearate to the mixture obtained in the above 2 to make the mixture uniform and avoid excessive lubrication.
[0104] 4. The theoretical tablet weight is 80 mg / tablet, and the tablet weight difference is controlled to be no more than 10%, the disintegration time limit is less than 15 minutes, and the friability is less than 1.0%.
[0105] 5.15% Opadry solution is used for film coating, and the tablet bed temperature...
Embodiment 3
[0106] Embodiment 3-direct compression method
[0107] The composition of the prescription is as follows: 80mg / tablet
[0108]
[0109]
[0110] Preparation method: with reference to Example 1 and Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com