Fluconazole ear drops and preparation method thereof
A fluconazole ear drop and fluconazole technology are applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. The effect of suitable fluidity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
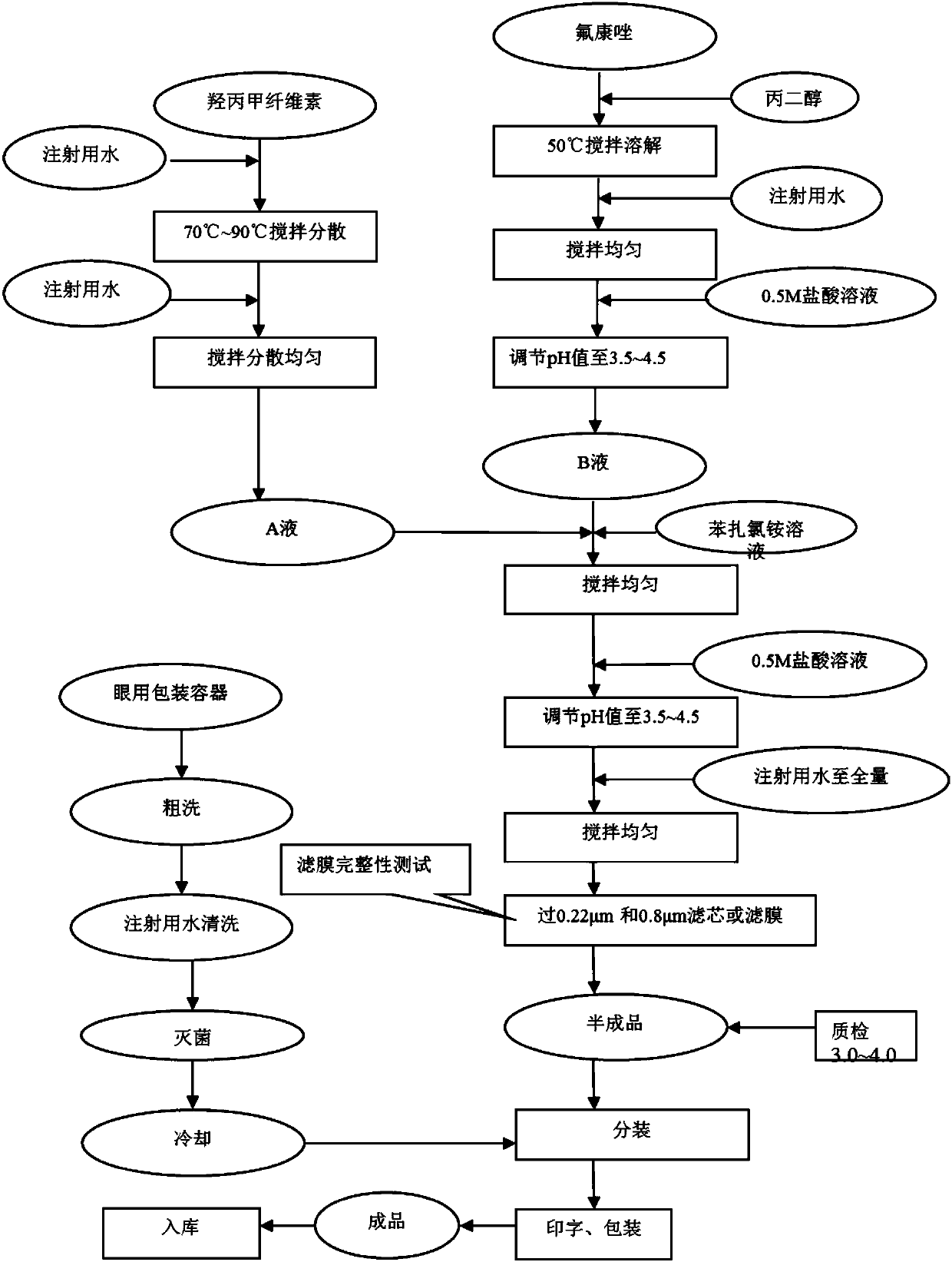
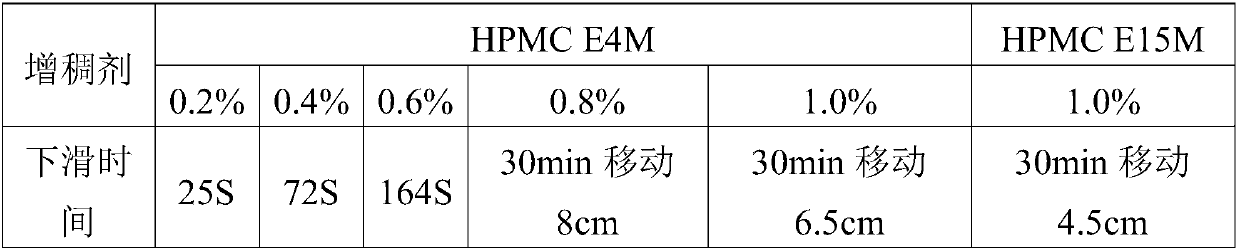
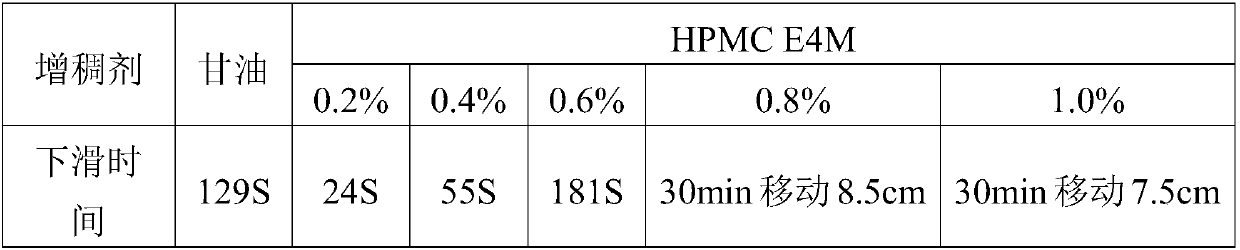
[0032] Fluconazole ear drops comprises the following components in weight percentage: 0.1% of fluconazole, 0.2% of hypromellose (HPMCE4M), 0.0025% of benzalkonium chloride and 8% of propylene glycol, and the balance is water for injection, pH is 3.5.
[0033] The preparation method of fluconazole ear drops comprises the steps:
[0034] (1) Dissolving the formulated amount of hypromellose in an appropriate amount of water for injection at 70°C and stirring to form a transparent solution to obtain solution A;
[0035] (2) Mix fluconazole and propylene glycol in the prescribed amount, stir and dissolve at 50°C, add an appropriate amount of water for injection, and adjust the pH to 3.5 with 0.5mol / L hydrochloric acid solution to obtain solution B;
[0036] (3) Mix solution A and solution B evenly, add benzalkonium chloride in the prescribed amount, stir evenly, adjust the pH to 3.5 with hydrochloric acid solution, add water for injection to the full amount, stir evenly, let stand...
Embodiment 2
[0038] Fluconazole ear drops, which comprises the following components in weight percentage: 1.0% fluconazole, 1.0% hypromellose (HPMCE4M), 0.01% benzalkonium chloride and 18% propylene glycol, and the remainder is water for injection , pH 4.5.
[0039] A preparation method of fluconazole ear drops, the method may further comprise the steps:
[0040] (1) Dissolving the formulated amount of hypromellose in an appropriate amount of water for injection at 90°C and stirring to form a transparent solution to obtain solution A;
[0041] (2) Mix fluconazole and propylene glycol in the prescribed amount, stir and dissolve at 50°C, add an appropriate amount of water for injection, and adjust the pH to 4.5 with 0.5mol / L hydrochloric acid solution to obtain solution B;
[0042] (3) Mix solution A and B solution evenly, add benzalkonium chloride in the formula amount, stir evenly, adjust the pH to 4.5 with hydrochloric acid solution, add water for injection to the full amount, stir evenl...
Embodiment 3
[0044] Fluconazole ear drops comprises the following components in weight percentage: 0.8% of fluconazole, 0.6% of hypromellose (HPMCE4M), 0.005% of benzalkonium chloride and 8% of propylene glycol, and the balance is water for injection, pH is 4.0.
[0045] The preparation method of fluconazole ear drops comprises the steps:
[0046] (1) Dissolving the formulated amount of hypromellose in an appropriate amount of water for injection at 80°C, stirring to form a transparent solution to obtain solution A;
[0047] (2) Mix the formulated amount of fluconazole and propylene glycol, stir and dissolve at 50°C, add an appropriate amount of water for injection, and adjust the pH to 4.0 with 0.5mol / L hydrochloric acid solution to obtain solution B;
[0048](3) Mix solution A and B solution evenly, add benzalkonium chloride in the formula amount, stir evenly, adjust the pH to 4.0 with hydrochloric acid solution, add water for injection to the full amount, stir evenly, let stand, filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com