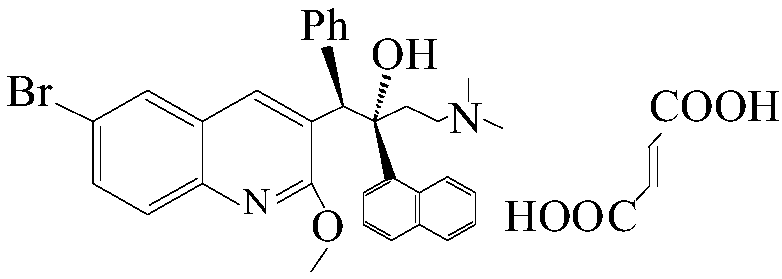
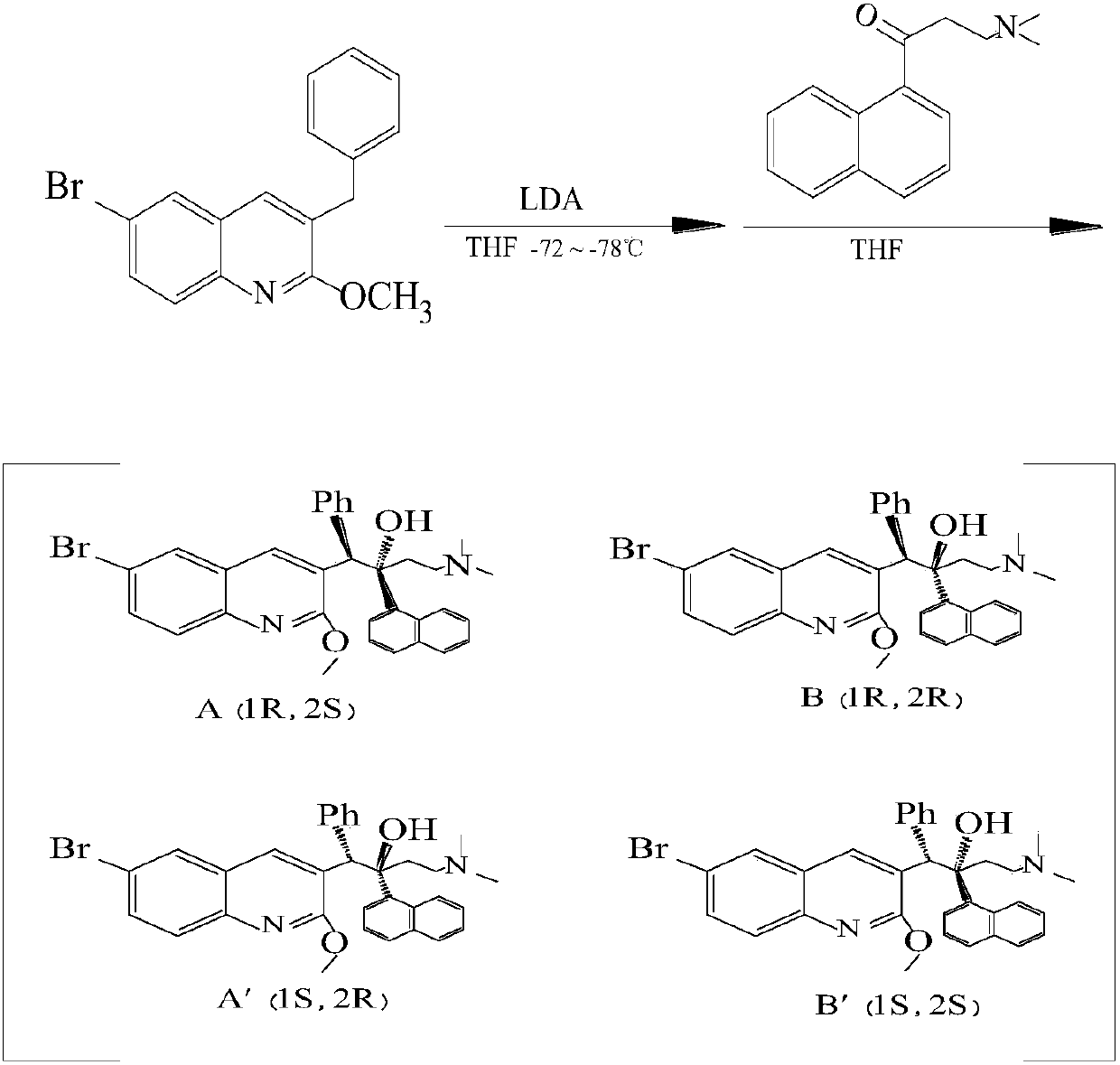
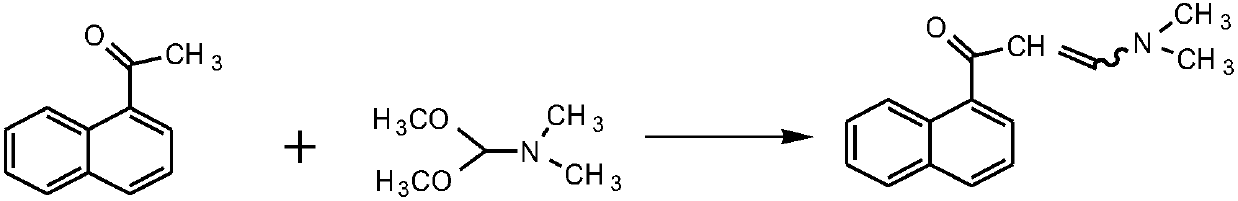
Method for preparing (1R, 2S)-bedaquiline and (1S, 2R)-bedaquiline
A bedaquiline, equivalent technology, applied in the field of preparation of and-bedaquiline, can solve the problems of many reaction steps, high manufacturing cost, increase of high-pressure hydrogenation steps, etc., achieve production efficiency improvement, less residual impurities, The effect of fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Add 30mL of anhydrous dichloromethane, 4.44g of (1R, 2R)-bedaquiline and (1S, 2S)-bedaquiline into a 500mL three-neck flask, blow nitrogen, stir and cool to -70℃~-78℃ Slowly add 1.36g of boron trifluoride·diethyl ether complex within 10min, keep the temperature constant, add 15mL of 2mol / L sodium hydroxide solution after 15min of reaction, raise the temperature to room temperature, and stir the solution at room temperature for 30min, Remove the water phase, wash the dichloromethane layer with 60mL of saturated sodium chloride solution, and then dry and concentrate the dichloromethane layer to obtain an oily product. The ratio of (B+B') is 62 / 38;
[0035] Then add 25mL of tetrahydrofuran to this oil, heat to reflux, and then cool to room temperature, a white solid and mother liquor are precipitated, the white solid is filtered off, and the filtered solid is (1R, 2R)-bedaquiline and (1S, 2S )-bedaquiline, after concentrating the mother liquor to obtain an oily substance, ...
Embodiment 2
[0046] Add 41mL of anhydrous dichloromethane, 5.99g of (1R,2R)-bedaquiline and (1S,2S)-bedaquiline mixture into a 500mL three-necked flask, blow nitrogen, stir and cool to -70°C~-78°C ℃, slowly add 3.71g of boron trifluoride·diethyl ether complex within 10min, keep the temperature constant, add 28mL of 2mol / L sodium hydroxide solution after 15min of reaction, raise the temperature to room temperature, and stir the solution at room temperature for 30min , remove the water phase, wash the dichloromethane layer with 80mL saturated sodium chloride solution, and then dry and concentrate the dichloromethane layer to obtain an oily product. The ratio of / (B+B') is 63 / 37;
[0047] Then add 25mL of tetrahydrofuran to this oil, heat to reflux, and then cool to room temperature, a white solid and mother liquor are precipitated, the white solid is filtered off, and the filtered solid is (1R, 2R)-bedaquiline and (1S, 2S )-bedaquiline, after concentrating the mother liquor to obtain an oil...
Embodiment 3
[0049] Add 36mL of anhydrous dichloromethane, 5.33g of (1R, 2R)-bedaquiline and (1S, 2S)-bedaquiline mixture into a 500mL three-neck flask, blow nitrogen, stir and cool to -70°C ~ -78°C ℃, slowly add 1.63g of boron trifluoride·diethyl ether complex within 10min, keep the temperature constant, add 18mL of 2mol / L sodium carbonate solution after 15min of reaction, raise the temperature to room temperature, and stir the solution at room temperature for 30min, Remove the water phase, wash the dichloromethane layer with 60mL of saturated sodium chloride solution, and then dry and concentrate the dichloromethane layer to obtain an oily product. The ratio of (B+B') is 62 / 38;
[0050] Then add 25mL of tetrahydrofuran to this oil, heat to reflux, and then cool to room temperature, a white solid and mother liquor are precipitated, the white solid is filtered off, and the filtered solid is (1R, 2R)-bedaquiline and (1S, 2S )-bedaquiline, after concentrating the mother liquor to obtain an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com