Electrochemical synthesis method of 2-substituted benzothiazole
A technology of benzothiazole and synthesis method, which is applied in the field of electrochemical synthesis of benzothiazole, and achieves the effects of high yield, good selectivity and simple system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
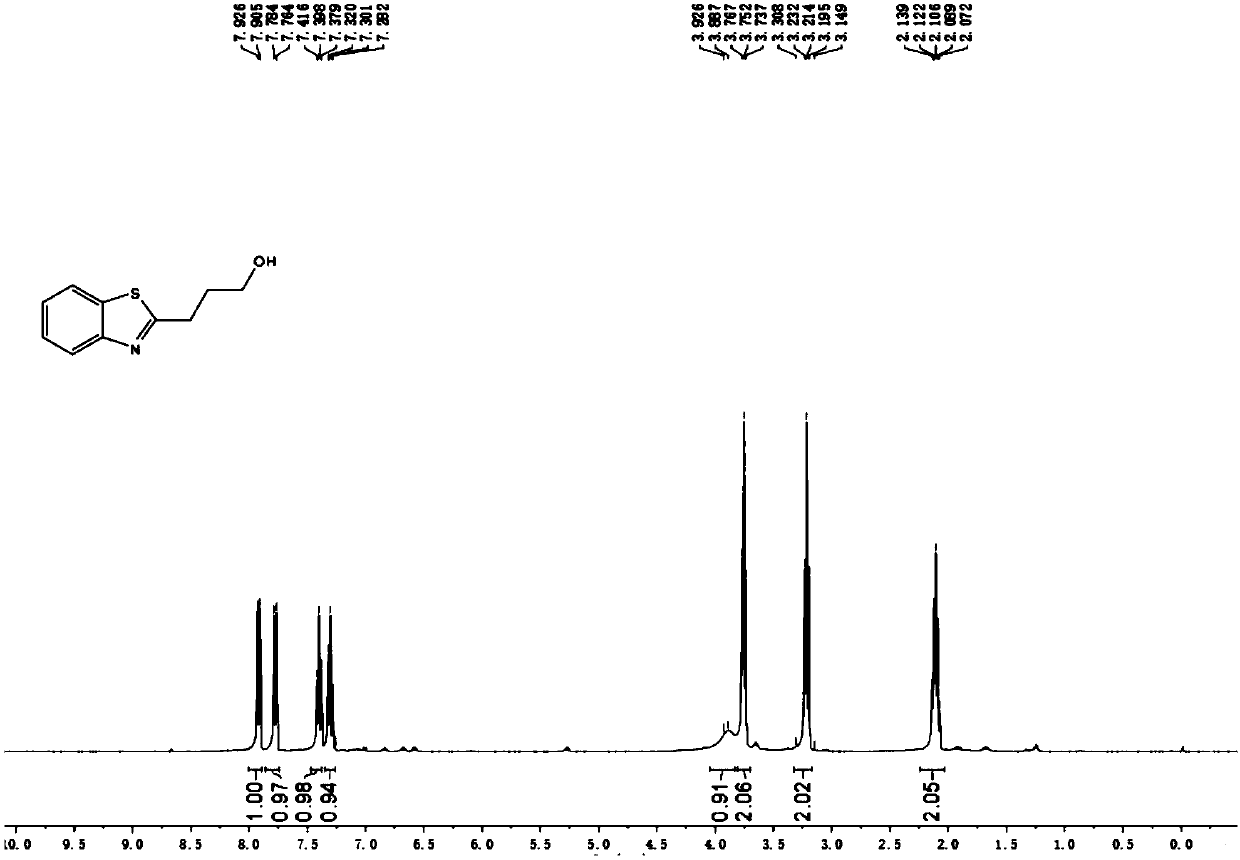
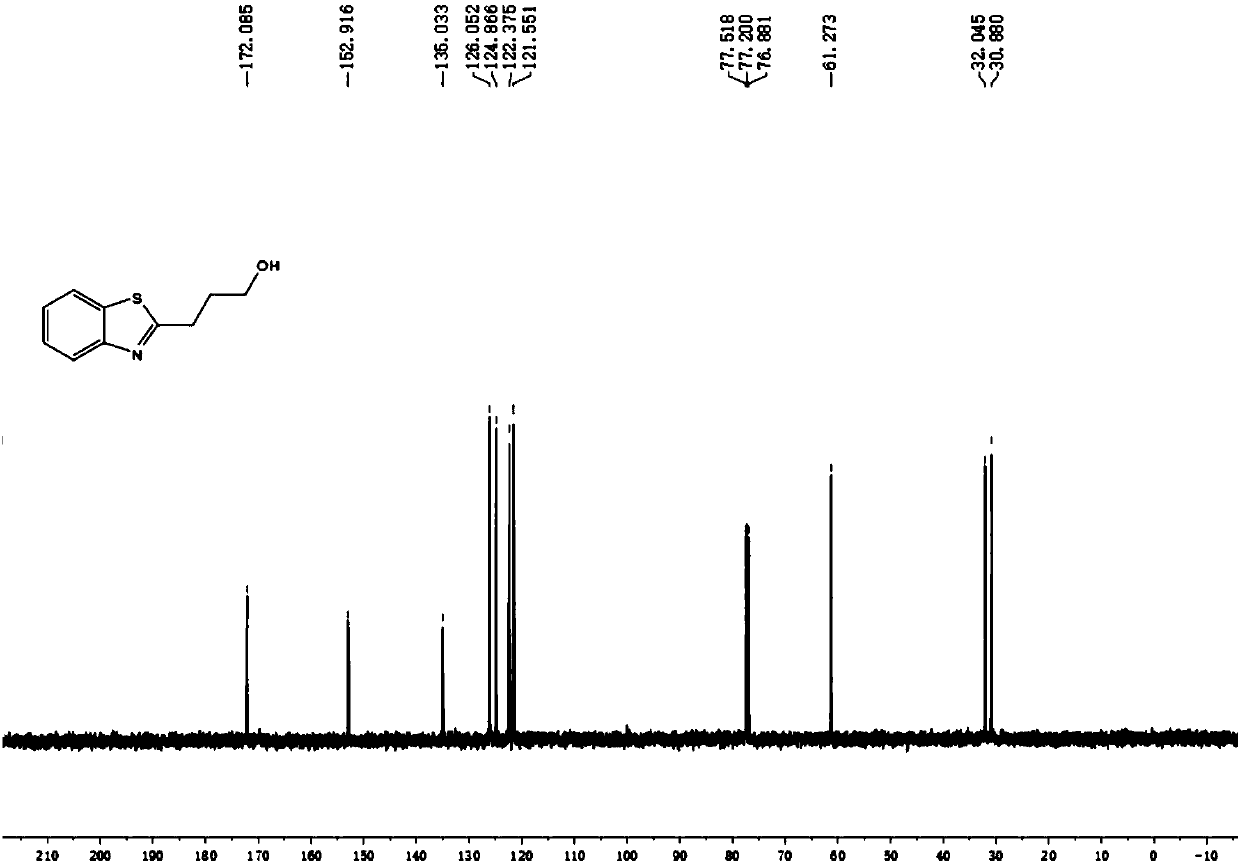
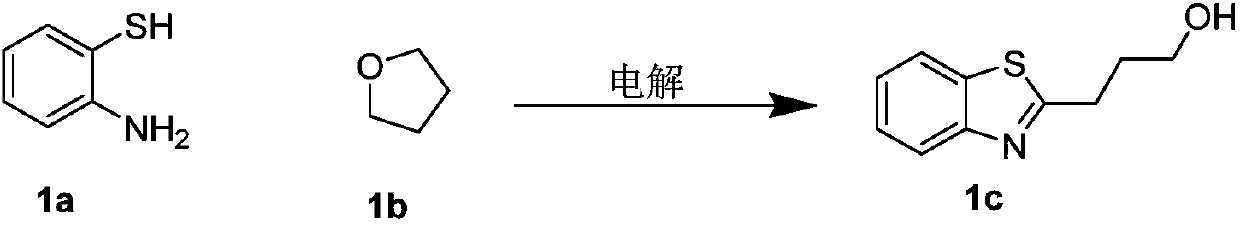
[0034] With metal platinum of φ10mm×15mm as the anode and cathode of the reaction, add 1.6mmol NaClO in turn to the round bottom flask 4 ·H 2 O, 0.0625mmol FeCl 3 , 0.25mmol 2-aminothiophenol, 5mL tetrahydrofuran, 600ul water, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 10mA, and electrolyze for 3.5h at room temperature. After the reaction, use ethyl acetate (15ml× 3) Extract the crude product, combine the organic layers, wash with saturated NaCl aqueous solution (10ml×1), anhydrous NaCl 2 SO 4 After drying and evaporating to dryness under reduced pressure, the product 3a was isolated with a yield of 68%.
[0035] The resulting product results from 1 HNMR, 13 CNMR OK.
Embodiment 2
[0037] With metal platinum of φ10mm×15mm as anode and metal copper of φ10mm×15mm as cathode, add 1.6mmol NaClO in turn to the round bottom flask 4 ·H 2 O, 0.0625mmol FeCl 3 , 0.25mmol 2-aminothiophenol, 5mL tetrahydrofuran, 600ul deionized water, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 10mA, and electrolyze for 3.5h at room temperature. After the reaction, use ethyl acetate ( 15ml×3) to extract the crude product, combine the organic layers, wash with saturated NaCl aqueous solution (10ml×1), anhydrous NaCl 2 SO 4 After drying and evaporating to dryness under reduced pressure, the product 3a was isolated with a yield of 53%.
[0038] The resulting product results from 1 HNMR, 13 CNMR OK.
Embodiment 3
[0040] With metal platinum of φ10mm×15mm as anode and metal nickel of φ10mm×15mm as cathode, add 1.6mmol NaClO in turn to the round bottom flask 4 ·H 2 O, 0.0625mmol FeCl 3 , 0.25mmol 2-aminothiophenol, 5mL tetrahydrofuran, 600ul deionized water, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 10mA, and electrolyze for 3.5h at room temperature. After the reaction, use ethyl acetate ( 15ml×3) to extract the crude product, combine the organic layers, wash with saturated NaCl aqueous solution (10ml×1), anhydrous NaCl 2 SO 4 After drying and evaporating to dryness under reduced pressure, the product 3a was isolated with a yield of 50%.
[0041] The resulting product results from 1 HNMR, 13 CNMR OK.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com