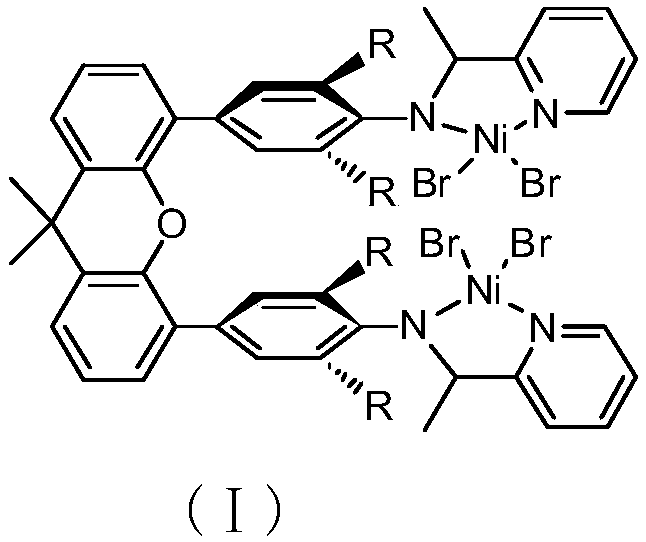
Binuclear xanthene bridging amino-pyridine nickel catalyst and preparation method and application thereof
A technology for bridging amine groups and nickel catalysts is applied in the field of catalysis, which can solve the problems of high reaction temperature and high production cost, and achieve the effects of high catalytic activity, low production cost and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
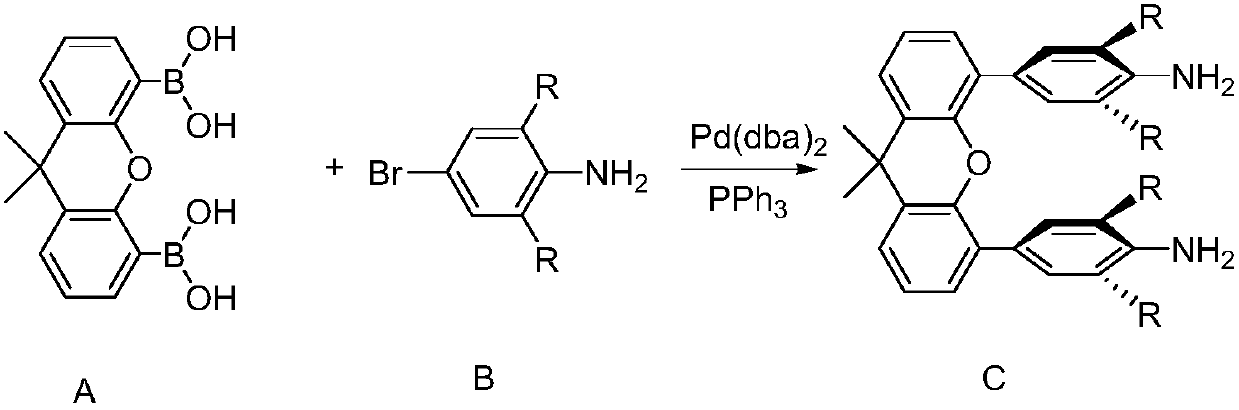
[0032] Synthesis of 9,9-dimethyl-9-xanthane-4,4′-bis[2-(2,6-diisopropyl)aniline-2-ethyl]pyridine, the reaction formula is as follows.
[0033]
[0034] Take xanthene diboronic acid (1.0g, 3.4mmol), 1,6-diisopropyl-p-bromoaniline (2.6g, 10.0mmol), sodium carbonate (1.0g, 9.4mmol), Pd (dba) 2 (0.2g, 0.35mmol), triphenylphosphine (0.1g, 0.4mmol), H 2 O (4mL), ethanol (7mL) and toluene (24mL) were mixed, stirred overnight at room temperature, after the reaction stopped, extracted with ethyl acetate, washed with sodium chloride, dried with magnesium sulfate, spin-dried the solvent, and added 10mL of methanol Recrystallized, filtered, and dried to obtain off-white solid 9,9-dimethyl-9-xanthene-4,4'-bis(2,6-diisopropyl)aniline (i.e., diamine compound C) (1.0g ,54%).
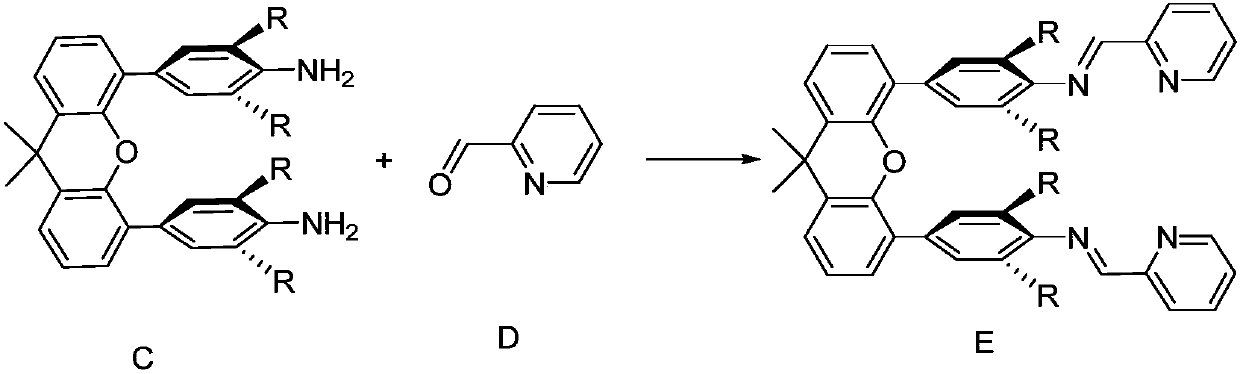
[0035] Take 9,9-dimethyl-9-xanthene-4,4'-bis(2,6-diisopropyl)aniline (1.0g, 1.79mmol), 2-pyridinecarbaldehyde (1.30g, 12.1mmol) And 0.05 equivalent of p-toluenesulfonic acid, dissolved in toluene (30mL), refluxed ...
Embodiment 2
[0038] Synthesis of 9,9-dimethyl-9-xanthane-4,4′-bis[2-(2,6-dimethyl)aniline-2-ethyl]pyridine, the reaction formula is as follows.
[0039]
[0040]
[0041] The procedure is the same as above, and 1,6-dimethyl-p-bromoaniline (2.0g, 10mmol) is added to finally obtain 9,9-dimethyl-9-xanthene-4,4'-bis[2-(2,6- Dimethyl)aniline-2-ethyl]pyridine, 84% (1.01 g). 1H NMR (500MHz, CDCl3, ppm): δ8.44(m, 2H, pydine-H), 7.33(m, pydine-H), 7.19(d, J=7.4Hz, 2H, pydine-H), 7.01( d,J=8.0Hz,4H,pydine-H,aryl-H),6.9(m,4H,aryl-H),6.80(s,4H,aryl-H),4.31(m,4H,CHCH3),3.94 (s,2H,NH),1.86(s,12H,aryl-CH3),1.53(m,6H,xanthene-CH3),1.36(m,6H,CHCH3);13C{1H}NMR(125MHz,CDCl3,ppm ):δ164.2,149.2,147.5,143.9,136.2,130.4,130.2,130.0,128.8,128.4,124.2,122.7,121.9,121.3,57.8(CHCH3),34.4(C(CH3)2),32.3(C(CH3) 2), 23.1 (CHCH3), 18.8 (aryl-CH3); HRMS (m / z): calcd for C45H47N4O: 659.3750; found: 659.4578 [M+H]+.
Embodiment 3
[0043] Synthesis of 9,9-Dimethyl-9-xanthene-4,4′-bis[2-(2,6-diisopropyl)aniline-2-ethyl]pyridine nickel complex (Ni-A) .
[0044]
[0045] Take (DME)NiBr 2 (0.17g, 0.54mmol) and 9,9-dimethyl-9-xanthen-4,4′-bis[2-(2,6-diisopropyl)aniline-2-ethyl]pyridine (0.21 g, 0.27mmol) into the Schlenk flask, add CH 2 Cl 2 (20 mL), the mixture was stirred at room temperature for 12 hours. The resulting suspension was filtered, the solvent was spin-dried, and the obtained powder was washed with diethyl ether (2×10 mL), and then vacuum-dried at room temperature to obtain a brown solid, which was Ni-A (0.21 g, 67%). Elemental analysis results: C, 52.78; H, 5.01; N, 4.65; found: C, 52.38; H, 4.69; N, 4.57; M–2Br]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com