Separating and recycling method for thallium in nickel-cobalt sulfate solution
A technology of separation and recovery of nickel and cobalt sulfate, applied in the direction of improving process efficiency, etc., can solve problems affecting the health of workers, affecting the extraction rate of thallium, environmental pollution, etc., and achieve low cost, improved extraction rate, and low operating conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
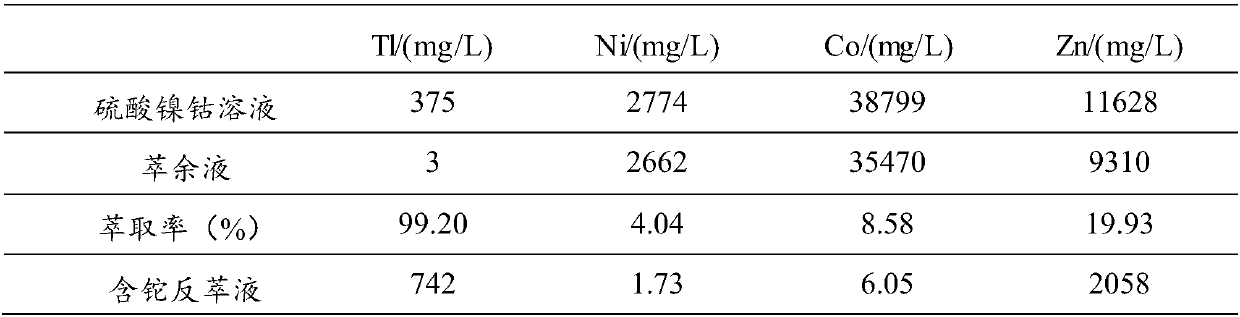
[0055] (1) Inject 1L of thallium-containing nickel-cobalt sulfate solution into the beaker, control the temperature of the nickel-cobalt sulfate solution to 25°C, turn on the stirring paddle, set the stirring speed to 340r / min, add NaOH solution to adjust the pH=1.5, adjust After completion, add 2 g mass concentration of 50% H 2 o 2 solution, and oxidized for 30 minutes to obtain a reaction mixture solution.
[0056] (2) add NaCl in the reaction mixed solution that step (1) obtains, the ratio of the add-on of NaCl and the volume of described reaction mixed solution is 50g: 1L, after stirring evenly, add extraction solvent (35%N 235 , 10% TBP, 55% sulfonated kerosene), extract according to O / A=1:1, set the stirring speed to 350r / min, stir for 3min, let stand for 10min, carry out two-phase separation after standing, and obtain Thallium-containing organic phase and raffinate (recovery of nickel and cobalt).
[0057] (3) Add 6mol / L of NH to the thallium-containing organic phase...
Embodiment 2
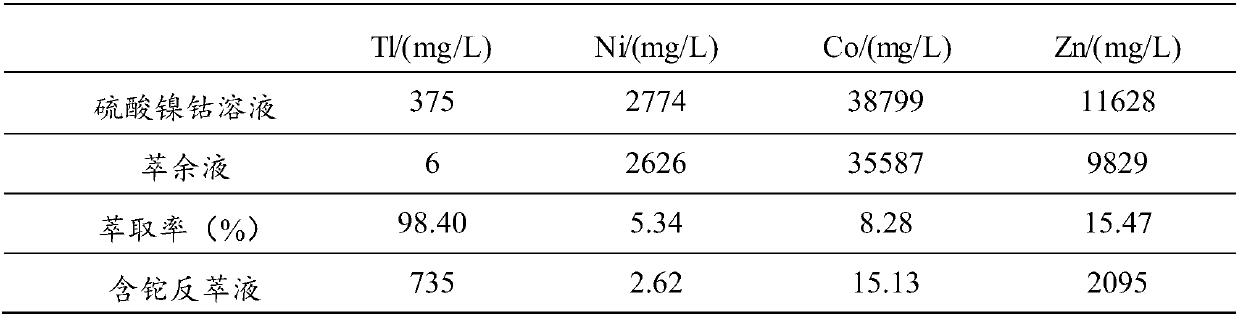
[0064] (1) Inject 1L of thallium-containing nickel-cobalt sulfate solution into the beaker, control the temperature of the nickel-cobalt sulfate solution to 25°C, turn on the stirring paddle, set the stirring speed to 340r / min, add NaOH solution to adjust the pH=1.5, adjust After completion, add 2 g mass concentration of 50% H 2 o 2 solution, and oxidized for 30 minutes to obtain a reaction mixture solution.
[0065] (2) add NaCl in the reaction mixed solution that step (1) obtains, the ratio of the add-on of NaCl and the volume of described reaction mixed solution is 10g: 1L, after stirring evenly, add extraction solvent (20% N 235 , 10% TBP, 70% sulfonated kerosene), extract according to O / A=1:1, set the stirring speed to 350r / min, stir for 10min, let stand for 10min, carry out two-phase separation after standing, and obtain Thallium-containing organic phase and raffinate (recovery of nickel and cobalt).
[0066] (3) Add 6mol / L of NH to the thallium-containing organic pha...
Embodiment 3
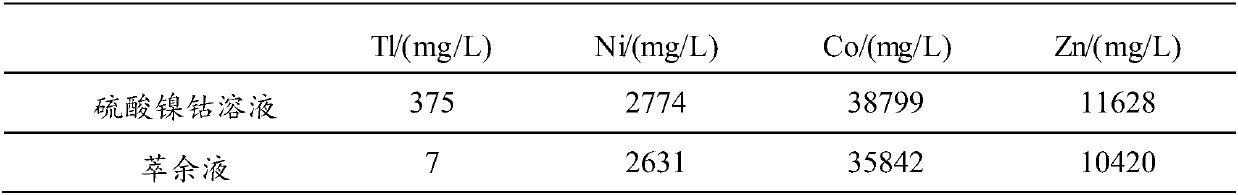
[0073] (1) Inject 1L of thallium-containing nickel-cobalt sulfate solution into the beaker, control the temperature of the nickel-cobalt sulfate solution to 25°C, turn on the stirring paddle, set the stirring speed to 350r / min, add NaOH solution to adjust the pH=1.5, adjust After completion, add 2 g mass concentration of 50% H 2 o2 solution, and oxidized for 30 minutes to obtain a reaction mixture solution.
[0074] (2) add NaCl in the reaction mixed solution that step (1) obtains, the ratio of the add-on of NaCl and the volume of described reaction mixed solution is 25g: 1L, after stirring evenly, add extraction solvent (5%N 235 , 10% TBP, 85% sulfonated kerosene), extract according to O / A=2:1, set the stirring speed to 350r / min, stir for 30min, let stand for 60min, carry out two-phase separation after standing, and obtain Thallium-containing organic phase and raffinate (recovery of nickel and cobalt).
[0075] (3) Add 6mol / L of NH to the thallium-containing organic phase o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com