Synthesis method of key raw material compound C of Elagolix
A synthesis method and compound technology, which is applied in the field of synthesis of Elagolix’s key raw material compound C, can solve the problems of unsuitability for large-scale industrial production, high synthesis cost, and uncommon raw materials, and achieve the goal of reducing synthesis cost, low cost and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A kind of synthetic method of the key raw material compound C of Elagolix, it comprises the following steps:
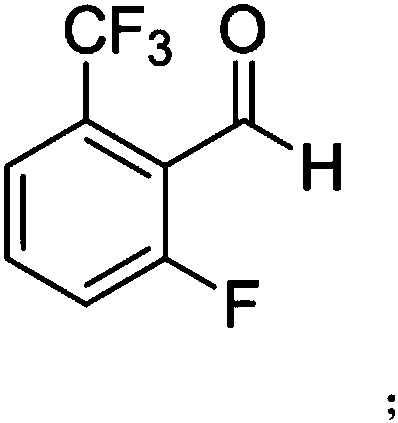
[0022] (1) Synthesis of intermediate A: 3-fluoro-trifluorotoluene reacts with organolithium reagents and DMF in the presence of tetramethylethylenediamine (TMEDA) and diisopropanolamine (DIPA) to obtain intermediate A ; Wherein, the structure of intermediate A is:
[0023]
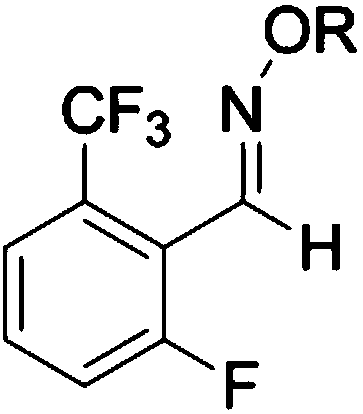
[0024] (2) Synthesis of intermediate B: intermediate A obtained in step (1) is oximated with alkoxyamine hydrochloride or hydroxylamine hydrochloride to obtain intermediate B; wherein, the structure of intermediate B is:
[0025]
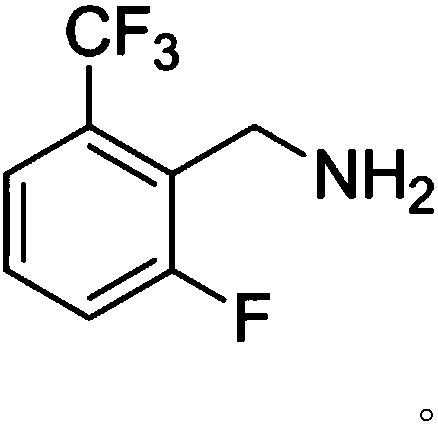
[0026] (3) Synthesis of compound C: the intermediate B obtained in step (2) is reduced to obtain compound C; wherein, the structure of compound C is:
[0027]
[0028] Wherein, the concrete operation method of step (1) is:
[0029] Dissolve 3-fluoro-trifluorotoluene, tetramethylethylenediamine, and diisopropanolamine in anhydrous organic solvent A, and...
Embodiment 1
[0041] 1.1 Synthesis of Intermediate A
[0042] In a 5L three-necked flask, add 3-fluoro-trifluorotoluene (492.0g, 3.0mol), TMEDA (383.2g, 3.3mol), DIPA (21.3g, 0.16mol) and anhydrous THF (1.5L), the system Cool down to -70~-78°C, slowly add n-BuLi (211.5g, 3.3mol) solution dropwise at -70~-78°C, react at this temperature for 2-3h; then slowly add DMF (328.7g ,4.5mol), continue to react at this temperature for 1-2h, then slowly rise to room temperature, and use saturated NH 4 Cl solution (500ml) was used to quench the reaction, and ethyl acetate (500ml×3) was used to extract and separate the liquids. The organic layers were combined, and the organic layers were concentrated and dried to obtain 510.1 g of solids with a yield of 88.7%.
[0043] 1 H-NMR (400MHz, DMSO-d6): 12.06 (1H, s), 7.80-7.72 (3H, m).
[0044] 1.2 Synthesis of Intermediate B
[0045] In a 3L three-neck flask, add Intermediate A (192.0g, 1.0mol), ethanol (500ml), NH 2 OH.HCl (82.8g, 1.2mol), NaOH (48g, 1....
Embodiment 2
[0053] 2.1 Synthesis of Intermediate A
[0054] In a 5L three-necked flask, add 3-fluoro-trifluorotoluene (492.0g, 3.0mol), TMEDA (522.5g, 4.5mol), DIPA (42.6g, 0.32mol) and anhydrous THF (2.0L), the system Cool down to -60~-70°C, slowly add n-BuLi (288.4g, 4.5mol) solution dropwise at -60~-70°C, and react at this temperature for 2-3h. Then slowly add DMF (328.7g, 4.5mol), continue the reaction at this temperature for 1-2h, then slowly rise to room temperature, 4 Cl solution (500ml) was used to quench the reaction, and ethyl acetate (500ml×3) was used to extract and separate the layers. The organic layers were combined, and the organic layer was concentrated and dried to obtain 460.6g of solid, with a yield of 80.1%.
[0055] 2.2 Synthesis of Intermediate B
[0056] In a 3L three-neck flask, add intermediate A (192.0g, 1.0mol), methanol (500ml), NH 2 OH.HCl (82.8g, 1.2mol), NaOAc (204.1g, 1.5mol) and H 2 O (300ml), then heated to 70-75°C, reacted at this temperature for 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com