Benzimidazole compound and derivatives, organic electronic transmission material as well as preparation of benzimidazole compound and derivatives and application of organic electronic transmission material
A technology of benzimidazole and transport materials, which is applied in the materials of organic semiconductor devices, organic chemistry, organic light-emitting devices, etc., to achieve the effect of enhancing rigid structure, enhancing rigidity, and enhancing long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
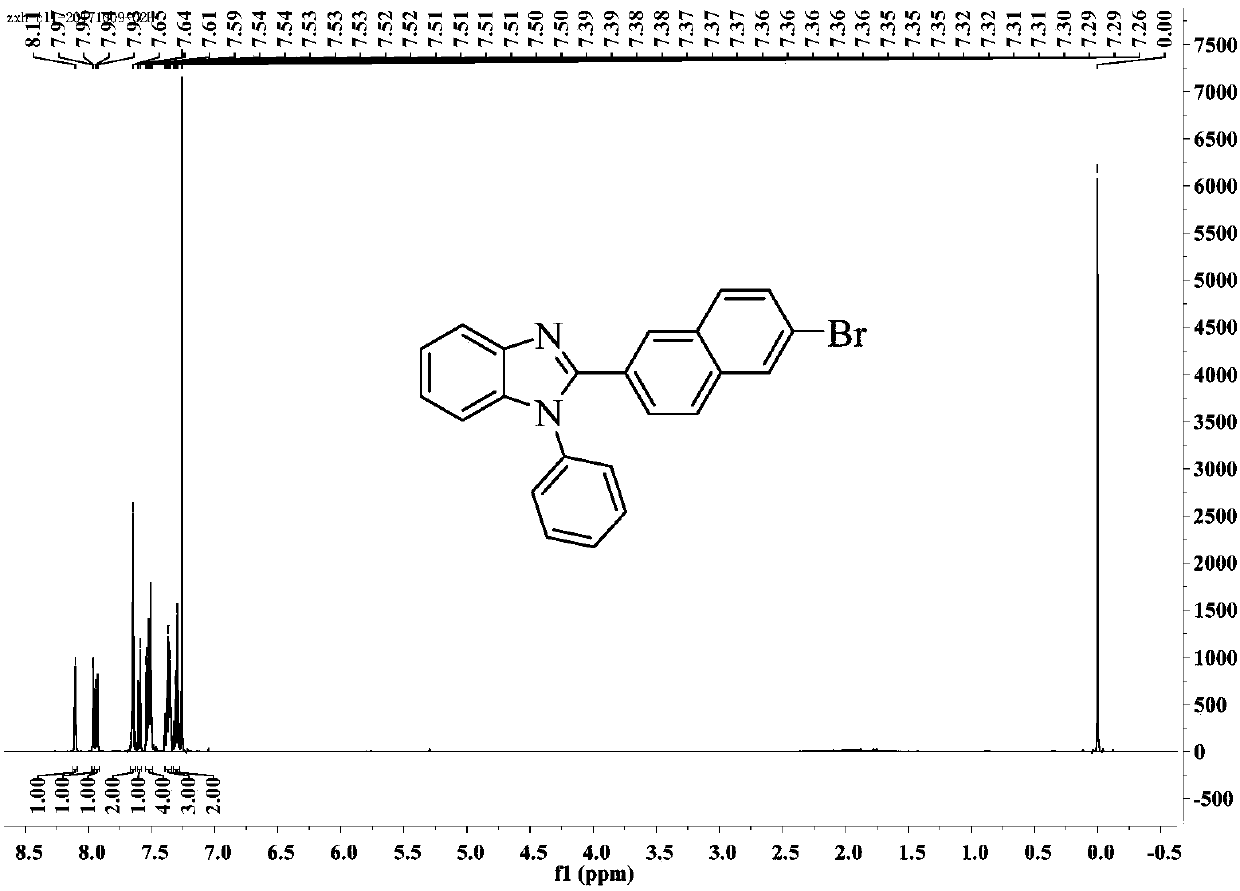
[0052] Embodiment 1 benzimidazole compound
[0053] A benzimidazole compound (2-(6-bromonaphthalene-2-yl)-1-phenyl-1H-benzimidazole), its structure is as follows:
[0054]
[0055] The preparation method of described benzimidazole compound comprises the following steps:
[0056] S1: Preparation of 6-bromo-2-naphthoyl chloride (1), the reaction equation is as follows:
[0057]
[0058] Under ice bath conditions, 6-bromo-2-naphthoic acid (5.0g, 19.91mmol) and thionyl chloride (14.2g, 119.48mmol) were added to a certain amount of chloroform (30ml), and the reaction was stirred at 60°C for 14 After ~16 hours, after the reaction was completed, chloroform was distilled off under reduced pressure to obtain 6-bromo-2-naphthoyl chloride (compound 1) as a white solid.
[0059] S2: Preparation of 6-bromo-N-(2-(phenylamino)phenyl)-2-naphthylcarboxamide (2), the reaction equation is as follows:
[0060]
[0061] 6-Bromo-2-naphthoyl chloride (compound 1) (5.36g, 19.91mmol) and o...
Embodiment 2
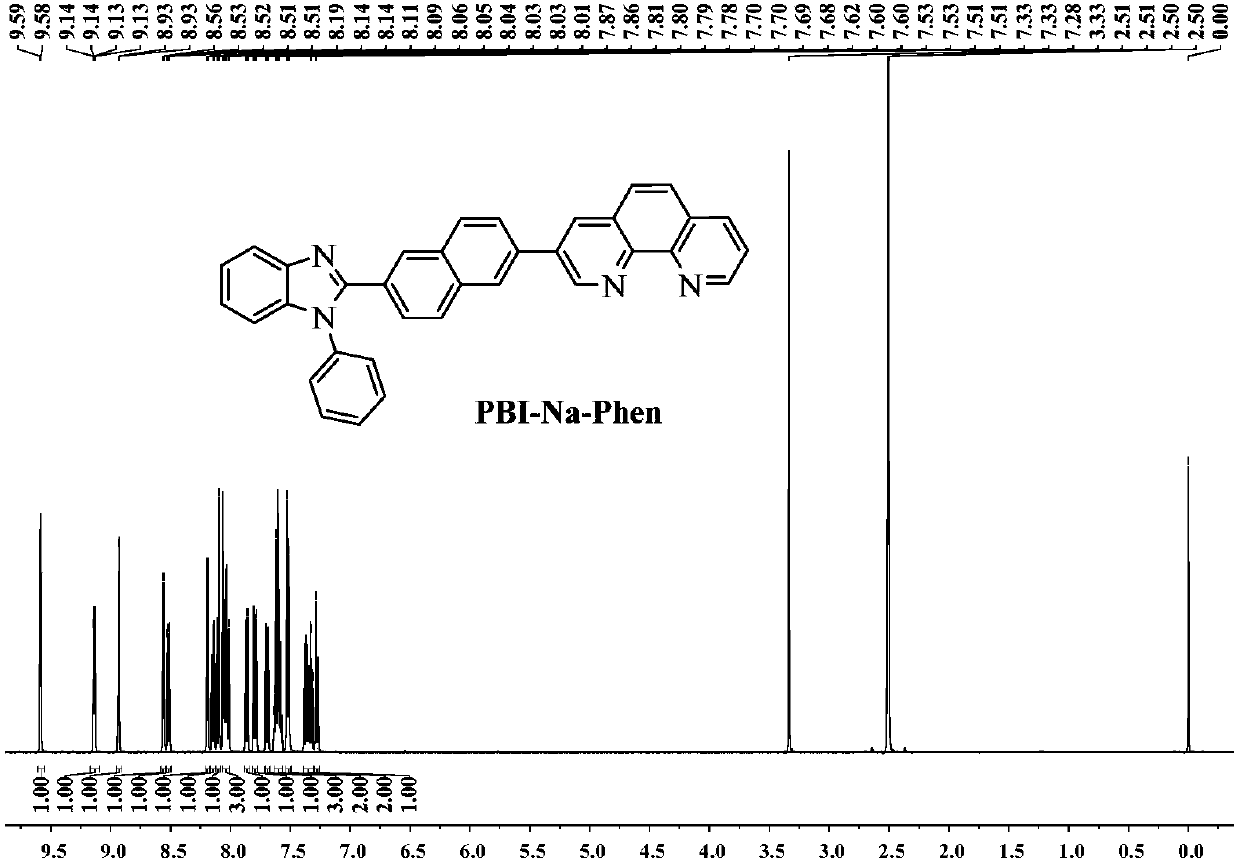
[0066] Example 2: Organic Electron Transport Materials
[0067] An organic small molecule electron transport material (PBI-Na-Phen) with high thermal stability and thin film morphology stability is a benzimidazole compound derivative with the following structure:
[0068]
[0069] The preparation method of described organic small molecule electron transport material (PBI-Na-Phen), comprises the following steps:
[0070] T1: 1-phenyl-2-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalen-2-yl)- The preparation of 1H-benzimidazole (4), reaction equation is as follows:
[0071]
[0072] 2-(6-bromonaphthalene-2-yl)-1-phenyl-1H-benzimidazole (compound 3) (3.0g, 7.51mmol) and biborpinacol ester (2.86g, 11.27mmol) and potassium acetate (2.21g, 22.53mmol) were added in a certain amount of tetrahydrofuran (60ml), under N 2 Under the atmosphere, add bistriphenylphosphine palladium dichloride (158mg, 0.225mmol), and stir the reaction at 80°C for 3-4 hours. After the reacti...
Embodiment 3
[0086] Embodiment 3 organic electron transport material
[0087] An organic small molecule electron transport material (PBI-Na-Trz) with high thermal stability and thin film morphology stability is a benzimidazole compound derivative with the following structure:
[0088]
[0089] The preparation method of described organic small molecule electron transport material (PBI-Na-Trz), comprises the following steps:
[0090] (1) 1-phenyl-2-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalene-2-yl) -1H-benzimidazole (compound 4): this step is the same as in Example 2;
[0091] (2) 2-(6-(4,6-diphenyl-1,3,5-triazin-2-yl)naphthalene-2-yl)-1-phenyl-1H-benzimidazole (PBI- Na-Trz) preparation, reaction equation is as follows:
[0092]
[0093] Compound 4 (1.4g, 3.15mmol) and 2-chloro-4,6-diphenyl-1,3,5-triazine (0.90g, 3.36mmol) were dissolved in toluene (50ml), and an appropriate amount of ethanol ( 6ml) and potassium carbonate aqueous solution (2M, 4ml, 8mmol), in N 2 U...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Crystallization temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com