A high stability two-dimensional cationic lead halide material and its preparation and application
A high-stability, cationic technology, applied in the field of fluorescent materials, can solve the problems of high LED quantum conversion efficiency, large demand for rare earth materials, aperture effect, moisture, air and heating instability, etc., to overcome self-absorption of luminescence And the effect of unstable luminous color, intact appearance and structure, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Material [PbF + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 Preparation and performance investigation:
[0040] Material [PbF + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 Preparation of:
[0041] Weigh 0.272g PbF 2 , 0.528g disodium adipate, dissolve it in 8mL water, measure 230mL perchloric acid and add the above solution, stir the obtained suspension for 30 minutes to make it evenly mixed, and transfer the uniform suspension to a volume of 12mL In the autoclave and sealed well, put it in an oven with a constant temperature of 150°C to react for 48 hours; after the reaction, take out and open the autoclave, transfer the obtained solid to a beaker, wash with 20mL of water and 20mL of ethanol in turn, and then dry it in the air , to get the material [PbF + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 .
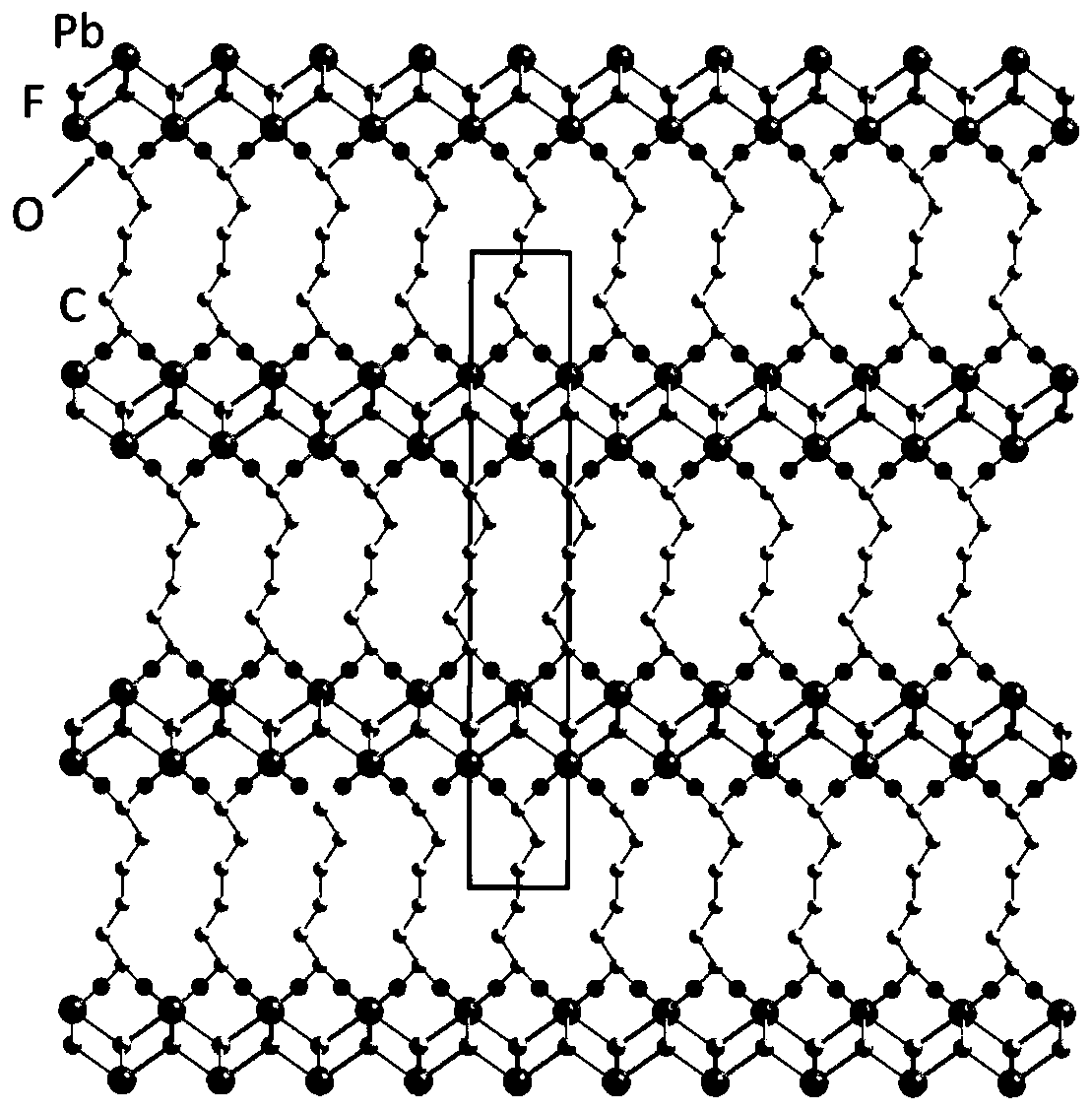
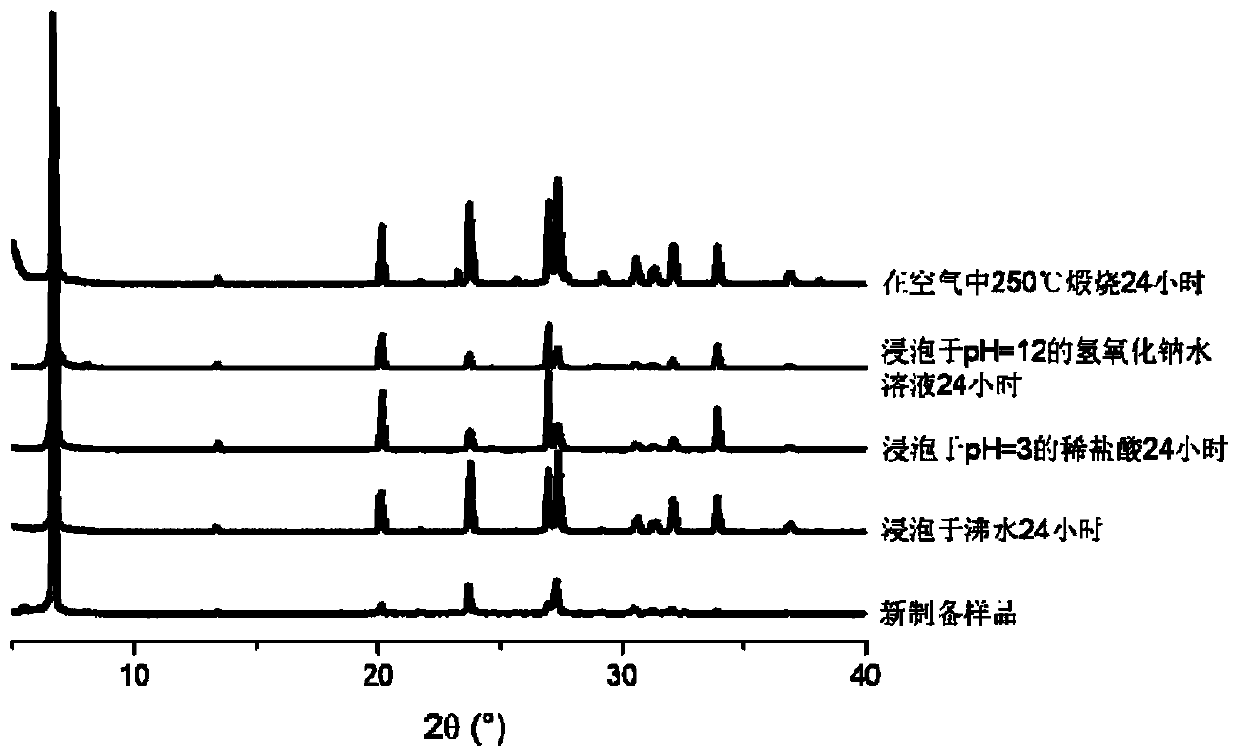
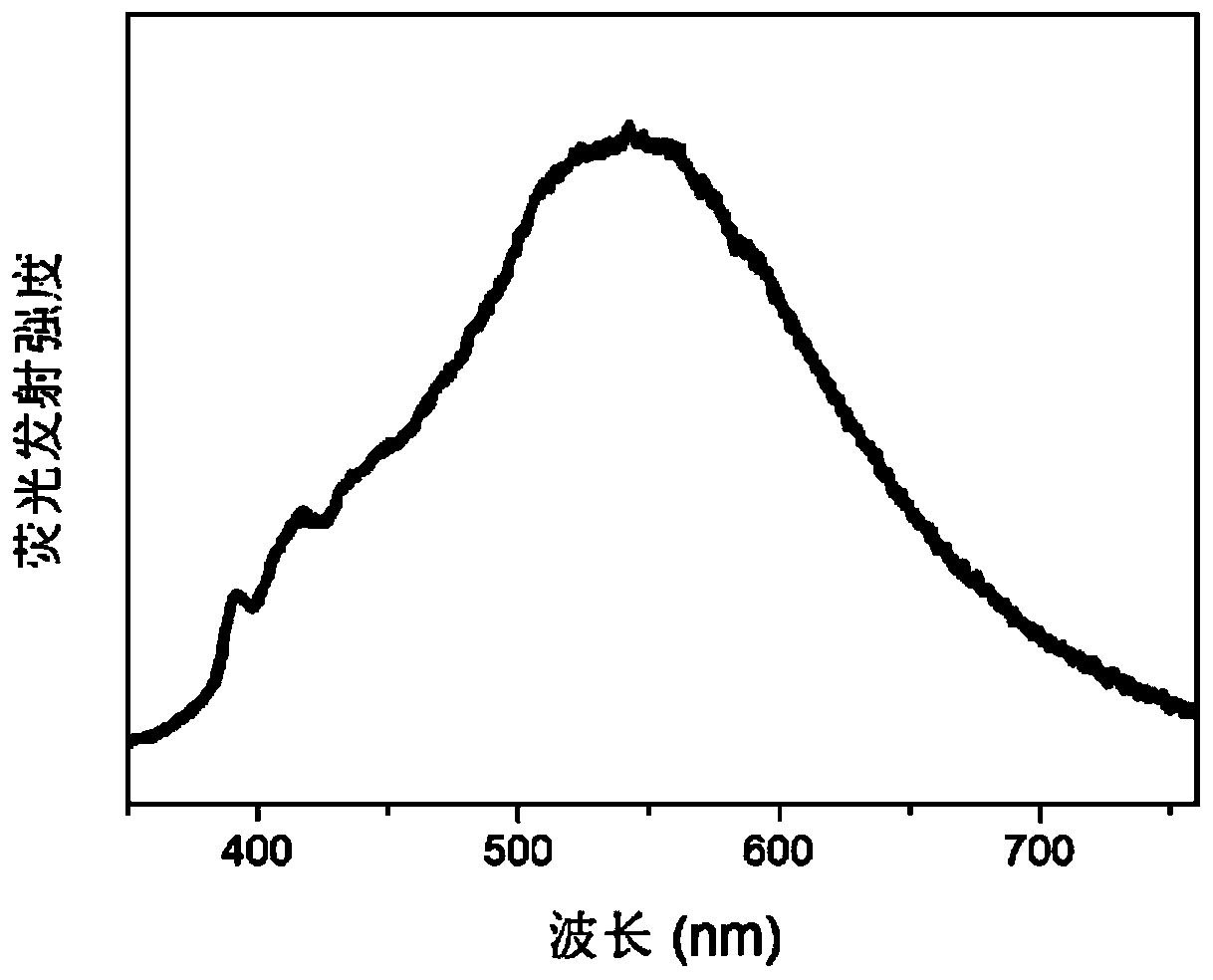
[0042] The properties of the material obtained in this embodiment are white crystals. Confirmed by X-ray single crystal diffraction characterization, the frame connection stru...
Embodiment 2
[0048] Material [PbCl + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 Preparation and performance investigation:
[0049] Material [PbCl + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 Preparation of:
[0050] Weigh 0.417g PbCl 2 , 0.570g disodium adipate, dissolve it in 8mL water, measure 430mL perchloric acid and add the above solution, stir the obtained suspension for 30 minutes to make it evenly mixed, and transfer the uniform suspension to a volume of 15mL In the autoclave and sealed well, put it in an oven with a constant temperature of 175°C to react for 48 hours; after the reaction, take out and open the autoclave, transfer the obtained solid to a beaker, wash with 20mL of water and 20mL of ethanol in turn, and then dry it in the air , to obtain the material [PbCl + ][ - o 2 C(CH 2 ) 4 CO 2 - ] 0.5 .
[0051] The properties of the material obtained in this embodiment are white crystals. Confirmed by X-ray single crystal diffraction characterization, the frame connecti...
Embodiment 3
[0057] Material [PbBr + ] 3 (H 2 O)[ - o 2 C(CH 2 ) 4 CO 2 - ] 1.5 Preparation and performance investigation:
[0058] Material [PbBr + ] 3 (H 2 O)[ - o 2 C(CH 2 ) 4 CO 2 - ] 1.5 Preparation of:
[0059] Weigh 0.551g PbBr 2 , 0.570g disodium adipate, dissolve it in 8mL water, measure 430mL perchloric acid and add the above solution, stir the resulting suspension for 30 minutes to make it evenly mixed, and transfer the uniform suspension to a volume of 20mL In the autoclave and sealed well, put it in an oven with a constant temperature of 175°C to react for 48 hours; after the reaction, take out and open the autoclave, transfer the obtained solid to a beaker, wash with 20mL of water and 20mL of ethanol in turn, and then dry it in the air , to obtain the material.
[0060] The properties of the material obtained in this embodiment are white crystals. Confirmed by X-ray single crystal diffraction characterization, the frame connection structure of the mater...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com