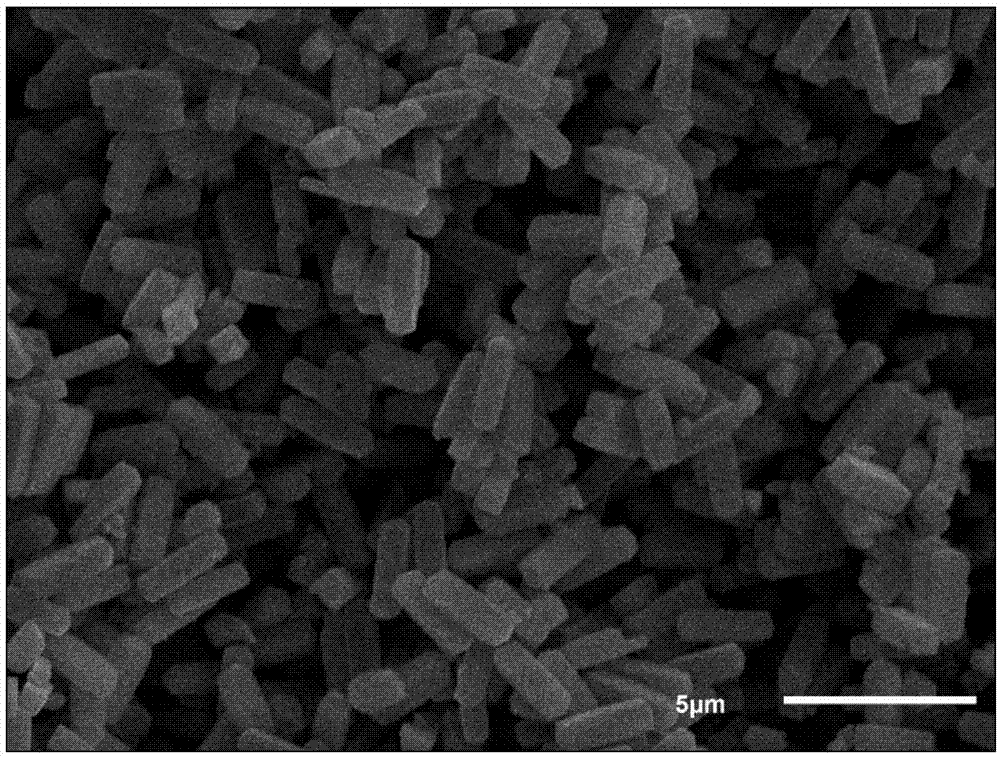
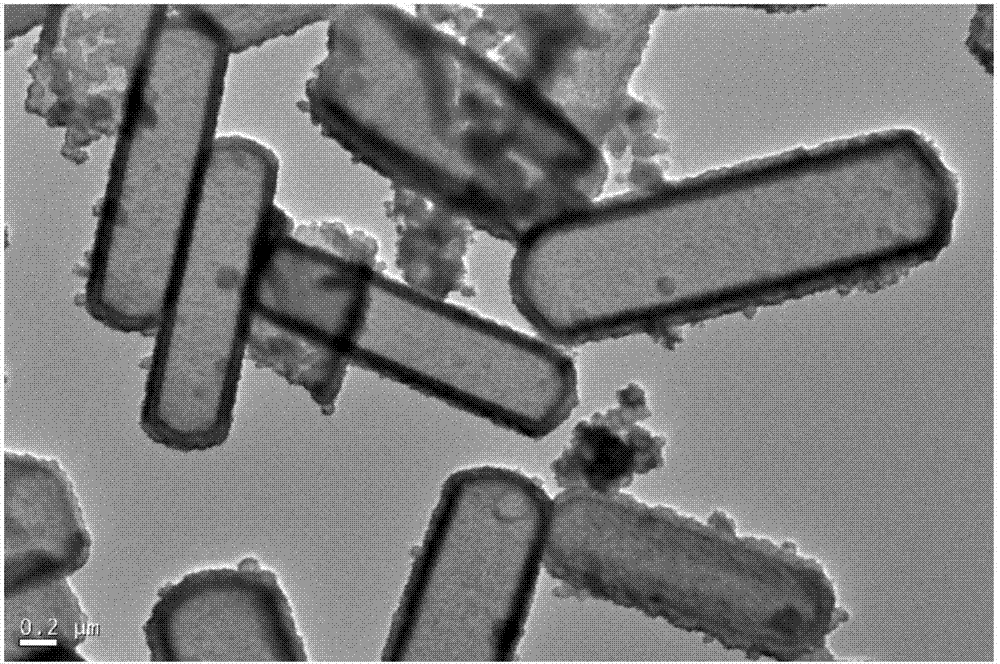
High-capacity carbon nanotube composite cobalt sulfide negative electrode material and preparation and application thereof
A carbon nanotube composite and negative electrode material technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problem of reducing the reversible capacity and cycle stability of electrode materials, volume expansion of electrode materials, structure collapse and other problems, to achieve the effect of improving reaction kinetics performance, electrochemical performance, and capacity fading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for preparing a high-capacity carbon nanotube composite CoS anode material for lithium-ion batteries includes the following steps:
[0051] 1. Preparation of cobalt sulfide:
[0052] (1) Dissolve 3 g of polyvinylpyrrolidone (PVP) uniformly in 200 mL of absolute ethanol under stirring conditions with a rotation speed of 500 rpm until the solution becomes clear.
[0053] (2) Under stirring conditions at a rotational speed of 600 rpm, 1.28 g of cobalt acetate tetrahydrate (Co(CH 3 COO) 2 ·4H 2 O) Add to the above solution, continue stirring at room temperature for 20 minutes, and let the cobalt acetate be uniformly dispersed and then stand for 48 hours to obtain the cobalt-containing precursor precipitate.
[0054] (3) Centrifuge the precipitated solution in step (2), remove the supernatant and wash with hot ethanol 10 times to obtain a cobalt-containing precursor.
[0055] (4) Put the cobalt-containing precursor obtained in step (3) into an oven at 60° C. and dry for 2 hou...
Embodiment 2
[0069] A method for preparing a high-capacity carbon nanotube composite CoS anode material for lithium-ion batteries includes the following steps:
[0070] 1. Preparation of cobalt sulfide:
[0071] (1) Dissolve 4 g of polyvinylpyrrolidone (PVP) uniformly in 200 mL of absolute ethanol under stirring conditions at 700 rpm, until the solution becomes clear.
[0072] (2) Under the condition of stirring at 500rpm, 1.5g cobalt acetate tetrahydrate (Co(CH 3 COO) 2 ·4H 2 O) Add to the above solution, keep stirring at room temperature for 30 minutes, and let the cobalt acetate be uniformly dispersed and then stand for 40 hours to obtain the cobalt-containing precursor precipitate.
[0073] (3) Centrifuge the precipitated solution in step (2), remove the supernatant, and wash 15 times with hot ethanol to obtain a cobalt-containing precursor.
[0074] (4) Put the cobalt-containing precursor obtained in step (3) into an oven at 80° C. for drying for 2 hours.
[0075] (5) Under the condition of cont...
Embodiment 3
[0085] A method for preparing a high-capacity carbon nanotube composite CoS anode material for lithium-ion batteries includes the following steps:
[0086] 1. Preparation of cobalt sulfide:
[0087] (1) Dissolve 4 g of polyvinylpyrrolidone (PVP) uniformly in 200 mL of absolute ethanol under stirring conditions with a rotation speed of 400 rpm until the solution becomes clear.
[0088] (2) Under the condition of stirring at 700rpm, 1g cobalt acetate tetrahydrate (Co(CH 3 COO) 2 ·4H 2 O) Add to the above solution, continue stirring at room temperature for 30 minutes, and let the cobalt acetate be uniformly dispersed and then stand for 48 hours to obtain the cobalt-containing precursor precipitate.
[0089] (3) Centrifuge the precipitated solution in step (2), remove the supernatant, and wash 15 times with hot ethanol to obtain a cobalt-containing precursor.
[0090] (4) Put the cobalt-containing precursor obtained in step (3) into an oven at 60° C. and dry for 4 hours.
[0091] (5) Under t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wall thickness | aaaaa | aaaaa |
| Capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com