Cu-OMS-2 catalyst capable of effectively removing formaldehyde in real environment and preparation method of Cu-OMS-2 catalyst
A real-environment, cu-oms-2 technology, applied in catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve problems such as unusable catalysts, achieve low manufacturing cost, large processing capacity, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for preparing a Cu-OMS-2 catalyst capable of effectively removing formaldehyde under a real environment, comprising the following steps:
[0031] (1) According to the molar ratio (5-40): (1-3): (0.5-1): (2-20) take bromoalkylimidazole ionic liquid, MnSO 4 ·H 2 O, KMnO 4 and CuSO 4 ·5H 2 O;
[0032] (2) Heat and melt the bromoalkylimidazolium ionic liquid first, then add MnSO 4 ·H 2 O, KMnO 4 and CuSO 4 ·5H 2 O, heat up to 180-240°C for 0.5-4h;
[0033] (3) After the reaction, the product is separated, washed and dried to obtain Cu-OMS-2 molecular sieve with regular nanofiber-like morphology.
Embodiment 2
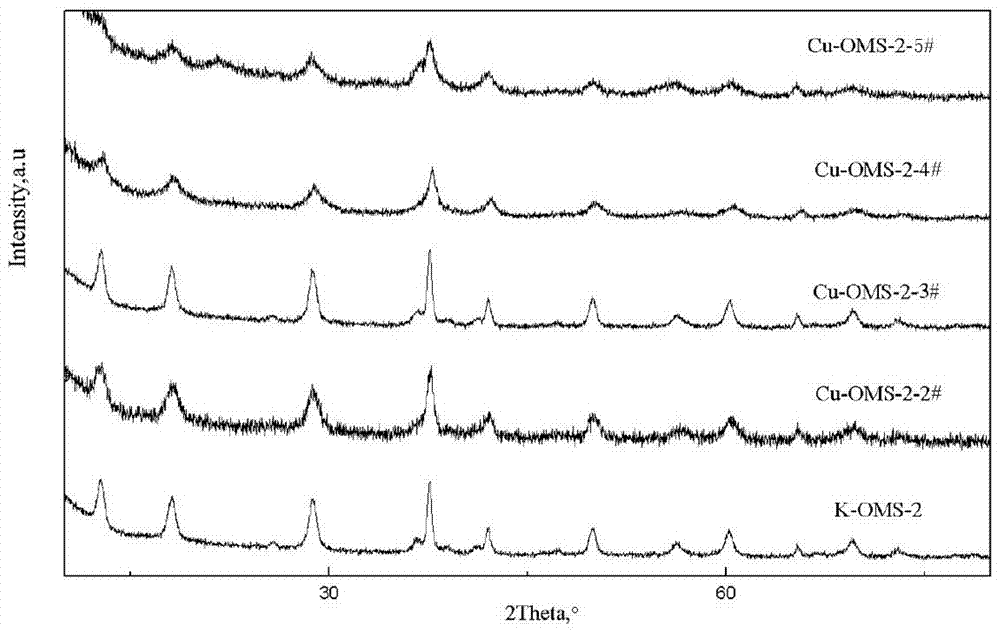
[0035] Weigh 150.211g (0.786mol) 1-ethyl-3-methylimidazolium bromide ionic liquid (molecular weight 191), 19.353g (0.114mol) MnSO 4 ·H 2 O (molecular weight 169), 14.762g (0.093mol) KMnO 4 (molecular weight 158), 189.886g (0.760mol) CuSO 4 ·5H 2 O (molecular weight 250). First, add 1-ethyl-3-methylimidazolium bromide ionic liquid into a 500ml three-neck flask, place it on an IKA oil bath magnetic stirrer, heat it to 100°C under the stirring condition of 500r / min, and then add MnSO 4 ·H 2 O, KMnO 4 and CuSO4 ·5H 2 O, then raise the temperature to 200°C within 30min and react for 1h. After the reaction, separate the solid product from the excess 1-ethyl-3-methylimidazolium bromide ionic liquid, wash the product with water and cold ethanol, and store the product at 110°C After drying for 12 hours, Cu-OMS-2 molecular sieve doped with copper was obtained. Its XRD results are attached figure 1 (marked as Cu-OMS-2-2#), SEM results are attached figure 2 , and the evaluation...
Embodiment 3
[0037] Weigh 220.561g (1.006mol) 1-butyl-3-methylimidazolium bromide ionic liquid (molecular weight 219.2), 20.887g (0.124mol) MnSO 4 ·H 2 O, 14.232g (0.090mol) KMnO 4 , 190.816g (0.763mol) CuSO 4 ·5H 2 O. First, add 1-butyl-3-methylimidazolium bromide ionic liquid into a 500ml three-necked flask, place it on an IKA oil bath magnetic stirrer, and heat it to 110°C under the stirring condition of 500r / min. After the temperature reaches, add MnSO 4 ·H2O 、KMnO 4 and CuSO 4 ·5H 2 O, then raise the temperature to 210°C within 40min and react for 1.5h. After the reaction, separate the solid product from the 1-butyl-3-methylimidazolium bromide ionic liquid, wash the product with water and cold ethanol, and dry it at 110°C for 12h , that is, Cu-OMS-2 molecular sieve doped with copper is obtained, and the XRD results are shown in the attached figure 1 (marked as Cu-OMS-2-3#), SEM results are attached image 3 , and the evaluation results of its catalytic oxidation performance t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com