Benzofuryl-containing acetylpiperazine compound and application thereof to medicine
A technology of benzofuryl and acetylpiperazine, which is applied in the field of compounds and its application in medicine, can solve problems such as difficult to control intestinal symptoms, and achieve the effect of simple synthesis method and inhibition of pancreatic lipase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
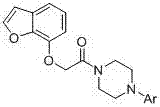
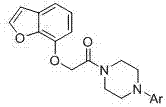
[0042] Example 1: Preparation of 1-[2-(benzofuran-7-oxyl)acetyl]-4-phenylpiperazine (compound number T01)
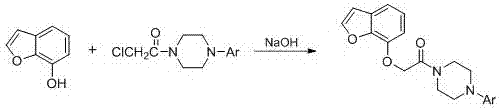
[0043] Add 4.0 g (0.03mol) of 7-hydroxybenzofuran, 9.4 g (0.03mol) of 4-phenyl-4-chloroacetylpiperazine, 0.8 g (0.005mol) of potassium iodide, and 13.8g (0.10mol) of potassium carbonate In the reaction flask, an appropriate amount of acetone was used as a solvent, and heated to reflux for 3.5 h. Suction filtration, rotary evaporation to remove acetone, add an appropriate amount of petroleum ether to dissolve, extract once with 5% NaOH aqueous solution, collect the organic layer and dry it with anhydrous magnesium sulfate. Filter, rotary evaporate to remove petroleum ether, and freeze to precipitate a white solid. Recrystallized from ethanol to obtain 5.2 g of white solid, yield 51.6%, mp: 123-124°C. ESI-MS m / z: 337.2; 1 H-NMR (CDCl 3 ) δ(ppm): 3.28-3.34(4H, m), 3.56-3.62 (4H, m), 4.86 (2H, s), 6.72(1H, d, J =8.4 Hz), 6.79-6.84(1H, m), 6.95(2H, d, J =6.6 Hz) , 7.06(...
Embodiment 2
[0044] Example 2: Preparation of 1-[2-(benzofuran-7-oxyl)acetyl]-4-(4-methylphenyl)piperazine (Compound No. T02)
[0045] According to the preparation method of Example 1, a white solid was obtained with a yield of 62.8%, mp: 134-136°C. ESI-MS m / z: 351.4; 1 H-NMR (CDCl 3 ) δ(ppm): 2.40 (3H, s), 3.27-3.35 (4H, m), 3.54-3.62 (4H, m), 4.85 (2H, s), 6.74(1H, d, J =8.4 Hz), 6.82 (2H, d, J = 6.6 Hz), 6.96(1H, d, J =7.2 Hz), 7.08 (2H, d, J = 6.6 Hz), 7.18(1H, d, J =7.2 Hz), 7.28-7.33(1H, m),7.68(1H, d, J =8.4 Hz); IR(KBr) υ / cm -1 : 3018, 2934, 1638, 1569, 1514, 1472, 1445, 1282, 1199, 1167, 1130, 1119, 1060, 834, 720, 652.
Embodiment 3
[0046] Example 3: Preparation of 1-[2-(benzofuran-7-oxyl)acetyl]-4-(2-methylphenyl)piperazine (Compound No. T03)
[0047] According to the preparation method of Example 1, a white solid was obtained with a yield of 45.6%, mp: 122-123°C. ESI-MS m / z: 351.4; 1 H-NMR (CDCl 3 ) δ(ppm): 2.36 (3H, s), 3.25-3.31 (4H, m), 3.58-3.64 (4H, m), 4.85 (2H, s), 6.56-6.62 (2H, m), 6.74(1H , m), 6.96(1H, d, J =7.2 Hz), 7.02(1H, d, J = 6.6 Hz), 7.08-7.12 (1H, d, J = 6.6 Hz), 7.18(1H, d, J =7.2 Hz),7.28-7.33(1H, m), 7.68(1H, d, J =8.4 Hz); IR(KBr) υ / cm -1: 3016, 2929, 1640, 1572, 1524, 1488, 1449, 1276, 1199, 1168, 1130, 1118, 1055, 834, 730, 649.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com