Medicinal composition and application thereof to preparation of drug for treating virus infection of flavivirus
A composition and medicine technology, applied in the field of medicine, to achieve the effect of enhancing therapeutic effect and treating flavivirus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] As an example of the pharmaceutical composition of the present invention, the pharmaceutical composition of this embodiment is: the pharmaceutical combination includes bortezomib and biotin, and the weight ratio of bortezomib and biotin is 1:10.
[0039]The bortezomib in this embodiment can be replaced by other boric acid 20S proteasome inhibitors, such as bortezomib pharmaceutically acceptable salts, exazomib or pharmaceutically acceptable salts thereof.
Embodiment 2
[0041] As an example of the pharmaceutical composition of the present invention, the pharmaceutical composition of this embodiment is: the pharmaceutical combination includes carfilzomib and biotin, and the weight ratio of carfilzomib and biotin is 1:2.5.
[0042] Carfilzomib in this embodiment can be replaced by other epoxyketone 20S proteasome inhibitors, such as a pharmaceutically acceptable salt of carfilzomib.
[0043] The medicine involved in the present invention can be prepared in any form, such as granule, powder, tablet, capsule, injection or oral liquid.
[0044] The medicament of the present invention may further include one or more pharmaceutically acceptable carriers or diluents, which will be properly formulated for administration, and the pharmaceutically acceptable diluents may be water, Ringer's solution, buffered saline ; The pharmaceutically acceptable carrier is glucose, maltodextrin, glycerin, mannitol, sorbitol, dextrin, lactose, gelatin, calcium sulfate...
Embodiment 3
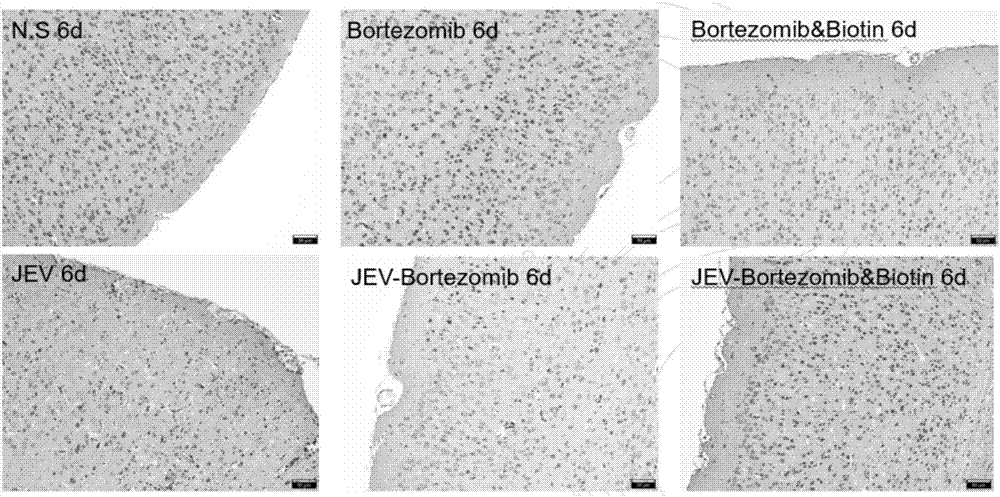
[0045] Example 3 Application of biotin combined with bortezomib in the treatment of mice infected with Japanese encephalitis virus
[0046] 1. Establishment of a mouse model infected with Japanese encephalitis virus
[0047] 1. Experimental materials:
[0048] (1) Experimental animals: four-week-old Balb / c female mice.
[0049] (2) Strain: JEV P3 strain.
[0050] (3) Other reagents:
[0051] 1) 0.01M PBS buffer: weigh NaH 2 PO4 0.593g, Na 2 HPO4 5.802g, NaCl 17.0g, deionized water (ddH 2 O) Dilute to 2L, store at room temperature for later use.
[0052] 2) DMEM basal medium: a bottle of DMEM powder, weighed NaHCO 3 3.7g, HEPES 5.95g, add 800mLddH 2 O, stir well to dissolve, dilute to 1000mL, sterilize with a 0.22μm filter, and store at 4°C for later use.
[0053] 2. Experimental steps
[0054] Mice were injected intraperitoneally with 10 6 PFU Japanese encephalitis virus, the mice in the control group were intraperitoneally injected with the same dose of DMEM mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com