Furan skeleton-containing imine guanidine derivatives and preparation and application thereof
A technology of guanidine furobisimide tetrahydrate and guanidine furobisimine, which is applied in the field of iminoguanidine derivatives and their preparation and application, and can solve the problems of high energy consumption for absorbent regeneration, strong corrosion of equipment, and easy degradation. problems, and achieve the effects of low cost, high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
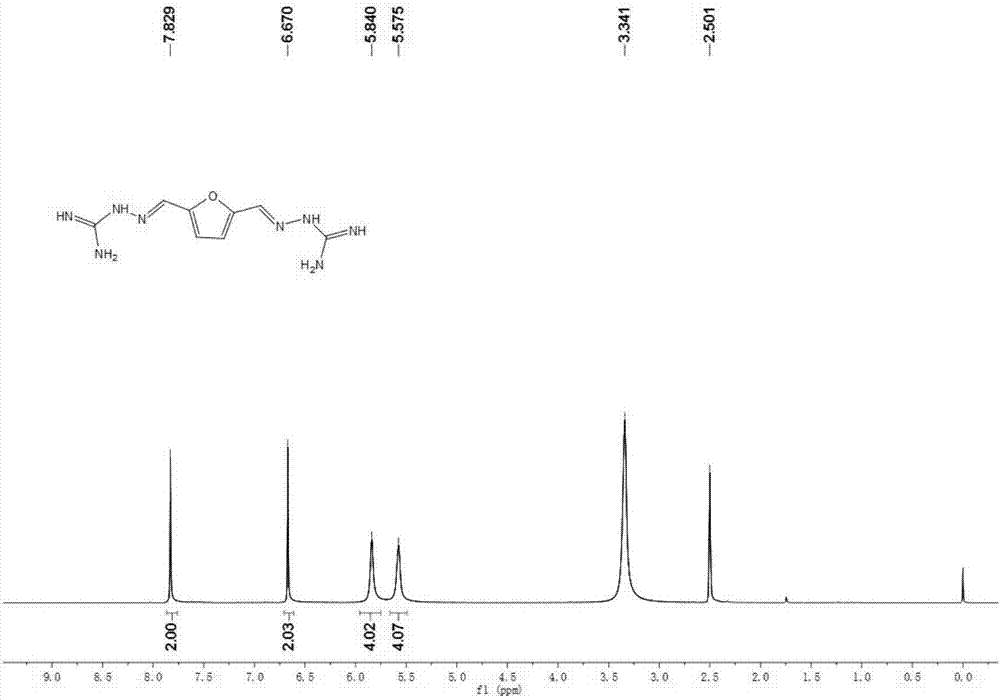
[0048] Embodiment 1: Preparation of 2,5-furanobisiminoguanidine
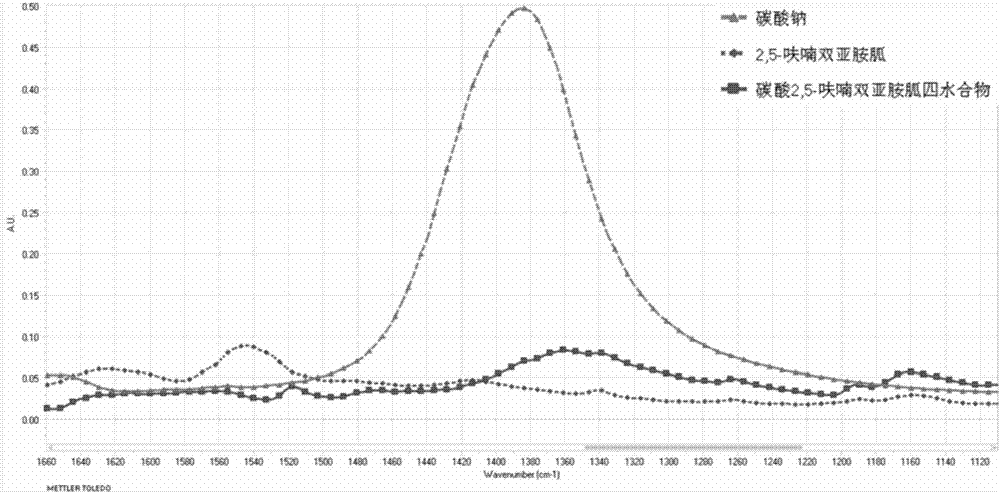
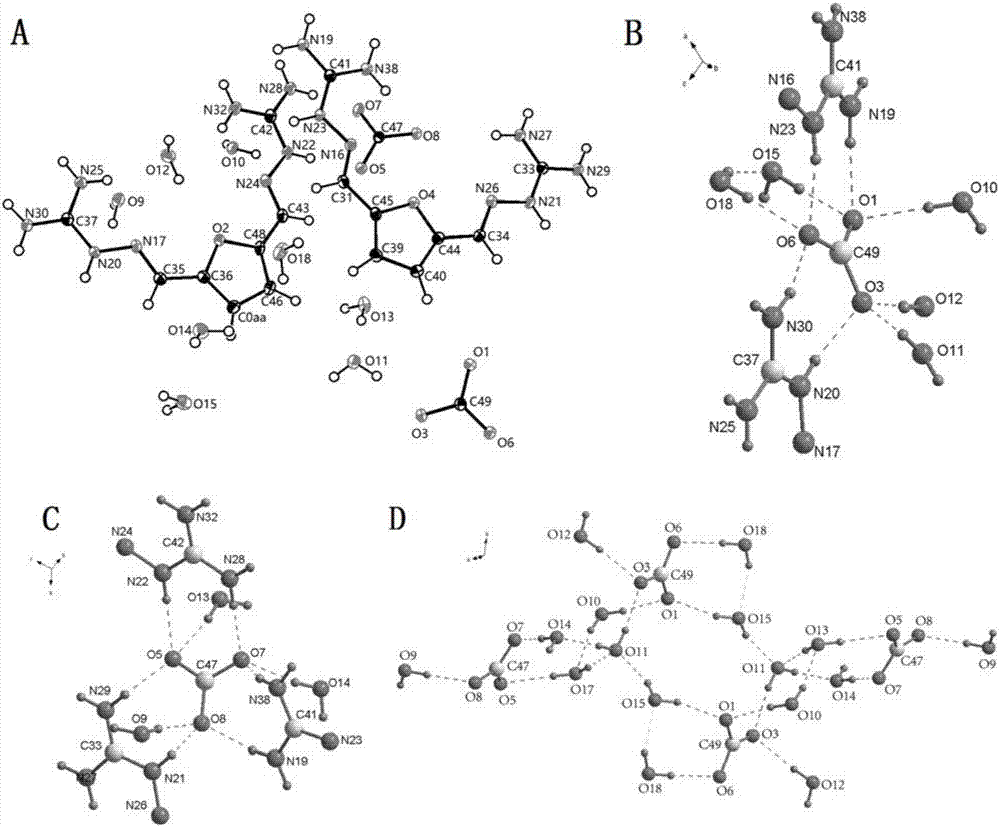
[0049] Add 2,5-furandicarbaldehyde (12.4g, 0.1mol), aminoguanidine hydrochloride (22g, 0.2mol), and ethanol (100ml) into the reaction flask, stir, heat to 70°C, and react for 12 hours. After the reaction was completed, the reaction solution was left to stand at 4°C for 12 hours, filtered with suction, and the filter cake was washed three times with ethanol, collected and dried to obtain 34.7 g of a light yellow solid, which was 2,5-furanobisiminoguanidine hydrochloride Salt hydrate, the chemical formula is FuBIG·2HCl·2.5H 2 O, yield 98.0%; mp.184-190°C; elemental analysis, theoretical value: C: 27.13%; H: 5.41%; N: 31.64%; measured value: C: 26.62%; H: 5.08%; N: 31.74%. Put it into a reaction bottle, add 100ml of 2M sodium hydroxide aqueous solution, stir at room temperature for 0.5 hours, stand at 4°C for 12 hours, filter with suction, and dry to obtain 22.66g of guanidine 2,5-furanobisimino, yield : 96%, me...
Embodiment 2
[0050] Embodiment 2: Preparation of 2,5-furanobisiminoguanidine
[0051] Add 2,5-furandicarbaldehyde (12.4g, 0.1mol), aminoguanidine hydrochloride (22g, 0.2mol), and tetrahydrofuran (100ml) into the reaction flask, stir, heat to 66°C, and react for 12 hours. After the reaction was completed, the reaction solution was left to stand at 4°C for 12 hours, filtered with suction, and the filter cake was washed three times with THF, the filter cake was collected, and dried to obtain a light yellow solid, which was 2,5-furanobisiminoguanidine hydrochloride . Put it into a reaction flask, add 100ml of 2M sodium hydroxide aqueous solution, stir at room temperature for 0.5 hours, let stand at 4°C for 12 hours, filter with suction, and dry to obtain 21.72g of 2,5-furanobisiminoguanidine, yield 92%, melting point: 244-246°C.
Embodiment 3
[0052] Embodiment 3: Preparation of 2,5-furanobisiminoguanidine
[0053] Add 2,5-furandicarbaldehyde (12.4g, 0.1mol), aminoguanidine hydrochloride (22g, 0.2mol), and methanol (100ml) into the reaction flask, stir, heat to 65°C, and react for 12 hours. After the reaction is completed, The reaction solution was left to stand at 4°C for 6 hours, filtered with suction, and the filter cake was washed three times with methanol, collected and dried to obtain a light yellow solid, which was 2,5-furanobisiminoguanidine hydrochloride. Put it into a reaction bottle, add 100ml of 2M sodium hydroxide aqueous solution, stir at room temperature for 0.5 hours, stand at 0°C for 10 hours, filter with suction, and dry to obtain 21.24g of 2,5-furanobisiminoguanidine, yield 90%, melting point: 244-246°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com