Catalyst for synthesizing methanol or its precursor, method for preparing the catalyst, and method for producing methanol or its precursor using the catalyst
一种合成甲醇、催化剂的技术,应用在有机化合物/氢化物/配位配合物催化剂、化学仪器和方法、物理/化学过程催化剂等方向,能够解决低选择性等问题,达到良好催化活性、确保长期使用、不易损失和分解的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Another aspect of the present invention relates to a method for the preparation of a catalyst comprising reacting a substituted or unsubstituted aniline with a Pt salt, as shown in the following reaction scheme:
[0055]
[0056] Another aspect of the present invention relates to a method for methane oxidation comprising (a) contacting a catalyst for synthesizing methanol or a precursor thereof according to any embodiment with methane in the presence of an acid.
[0057] As previously mentioned, the catalyst for synthesizing methanol or its precursor represented by one of Formulas 1 to 3 according to the present invention may be used to form methyl ester through esterification to realize methane oxidation. The methyl esters can be used to form functional derivatives by subsequent reaction with nucleophiles.
[0058] Specifically, methyl esters can react with water as a nucleophile to synthesize methanol as a functional derivative. Methyl esters can also be reacted w...
Embodiment 1
[0079] Embodiment 1: the synthesis of catalyst compound
[0080] (1) Synthesis of compound 1-1
[0081] Bis(aniline)dichloroplatinum
[0082]
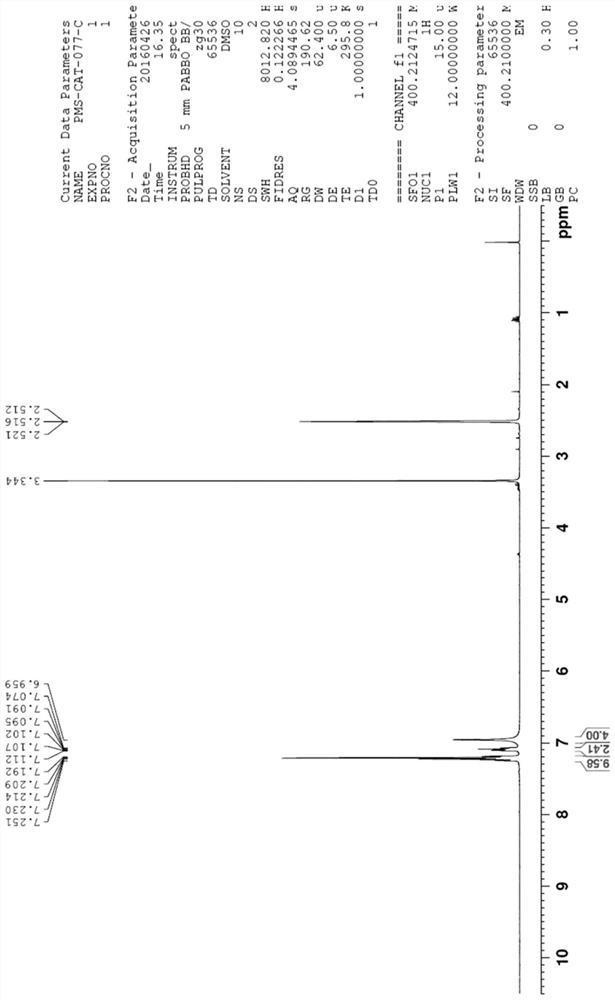
[0083] Will K 2 PtCl 4 (415 mg, 1.0 mmol) was added to an aqueous solution of aniline (502 mg, 5.4 mmol). After stirring well at room temperature for 18 hours, the precipitate was collected by filtration and washed with water and diethyl ether. The resulting solid was dissolved in dimethylformamide (DMF). The solution was stirred at 80°C for 3 hours. The reaction solution was concentrated under reduced pressure and precipitated with ether. The precipitate was collected by filtration to give the desired product (105 mg, 4.0 mmol) in 23% yield. 1 H NMR (400MHz, DMSO-d 6 )δ7.24-7.20 (m, 8H), 7.12-7.08 (m, 2H), 6.96 (s, 4H) (see image 3 )
[0084] (2) Synthesis of compound 1-2
[0085] Dichlorobis(4-methylaniline)platinum
[0086]
[0087] The desired product was obtained in a yield of 37% in the same manner as compoun...
Embodiment 2
[0132] Example 2: Synthesis of Methanol Precursor and Methanol
[0133] (1) Synthesis of methyl hydrogen sulfate
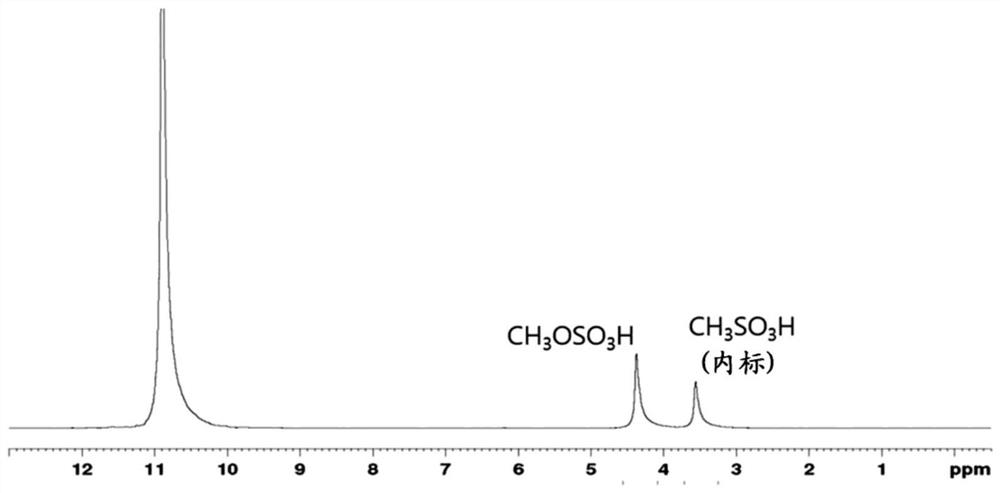
[0134] 1mg (2.6×10 -3 mmol) catalyst (dichloro-(N,N,N,N-tetramethylethylenediamine) platinum) represented by formula 3-2 and 30g containing 20% by weight SO 3 The oleum was mixed in a glass lined 100ml Inconel autoclave. The reactor was filled with methane gas to a pressure of 20 bar. The methane-filled reactor was heated to 180°C and the reaction was allowed to proceed for 3 hours. The pressure of methane at 180° C. was 35 bar at the initial stage of the reaction and decreased to 30 bar after 3 hours of reaction. After the reaction is complete, use a solution containing methanesulfonic acid (CH 3 SO 3 H) D as internal standard 2 SO 4 pass 1 H-NMR spectroscopy determines the structure of the product (see figure 1 ).
[0135] figure 1 Production of 1.89 g (16.9 mmol) of methyl hydrogensulfate was confirmed. The turnover number (TON) and turnover fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com