Method for preparing biphenyl-4-formyl corey lactone by using one-pot method
A technology of collidone diol and formyl family, which is applied in the field of chemical drug preparation, can solve the problems of low purity of intermediate products, unfavorable industrialized production, long reaction period and the like, and achieves improved product purity, less impurities and higher yield improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
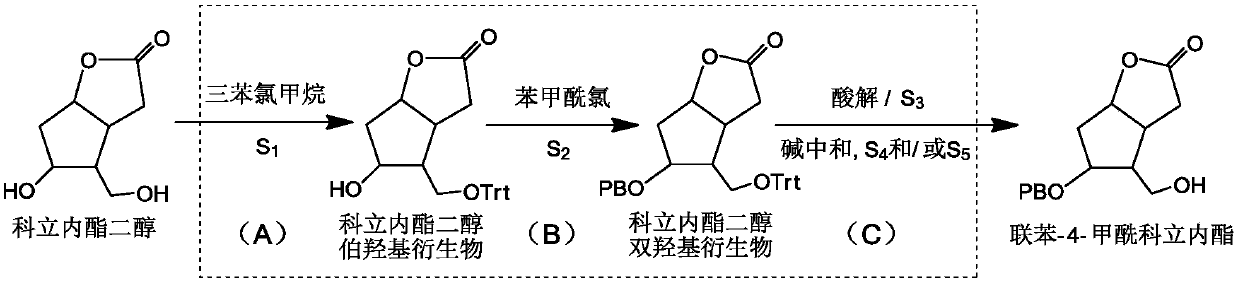
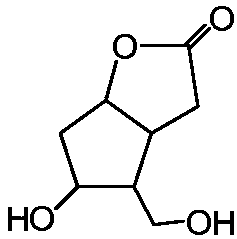
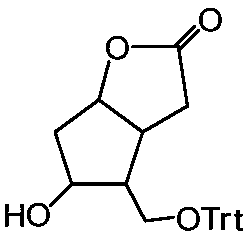
[0048] (A) Preparation of Corylidene Diol Primary Hydroxyl Derivatives: Add Solvent S to a Reactor with Filter Element at the Bottom 1 , add corylactone diol, stir until dissolved, then add triphenylchloromethane to carry out the alkylation reaction of the primary hydroxyl position, and stir the reaction for 6-12h under the condition of temperature control at 10-30°C until the reaction is complete. Solvent S for Lilactone Diol Primary Hydroxyl Derivatives 1 reaction system;
[0049] (B) preparation of the diol derivatives of corylidene diol: the solvent S obtained in the step (A) 1 Under the temperature control of the reaction system at 0-30°C, add biphenyl-4-formyl chloride dropwise to carry out the acylation reaction at the secondary hydroxyl position, and stir for 1-6 hours until the reaction is complete; add pure water and stir for 1-3 hours, and inject nitrogen , press filter, add solvent S 2 , refluxed for 1-4 hours, cooled to room temperature, and press-filtered to o...
Embodiment 1
[0110] Embodiment 1: (+) one-pot method prepares biphenyl-4-formyl corilide
[0111] Add 5L of pyridine to the reactor with a filter element at the bottom, add (+) corylactone diol 1.0kg (1eq) under stirring, add 1.62kg (1eq) triphenylchloromethane, and control the temperature at 10°C for 12h until complete To react, add 1.26kg (1eq) of biphenyl-4-formyl chloride, control the temperature at 0°C and react for 6h until complete reaction; add 10L pure water to the reaction kettle and stir for 3h to obtain a suspension. The filter element at the bottom will be suspended and hydraulically filtered, then add 5L of methanol to the reactor, heat and reflux for 2h, press filter the liquid to obtain solid powder; add 10L of acetonitrile to the reactor, add 7L (6mol / L) hydrochloric acid aqueous solution, and control the temperature React at 30°C for 12 hours until complete reaction, lower the temperature of the reaction system to 0°C, add saturated aqueous sodium bicarbonate solution to ...
Embodiment 2
[0112] Embodiment 2: One-pot method prepares (-) biphenyl-4-formyl corilide
[0113] Add 8.8L of pyridine to the reactor with a filter element at the bottom, add (-) corylactone diol 0.8kg (1eq) under stirring, add 1.56Kg (1.2eq) triphenylchloromethane, and react at a temperature of 30°C for 6h To complete the reaction, add 1.11Kg (1.1eq) biphenyl-4-formyl chloride, and react at a temperature of 30°C for 2 hours until the reaction is complete; add 8.8L of pure water to the reaction kettle and stir for 2 hours to obtain a suspension, which is passed into the reaction kettle Nitrogen, through the filter element at the bottom of the kettle to hydraulically filter the suspension, then add 5L of ethanol to the reaction kettle, heat and reflux for 2h, press filter the liquid to obtain a solid powder; add 8L tetrahydrofuran to the reaction kettle, and drop 7L (4mol / L) Aqueous hydrochloric acid, temperature controlled at 60°C for 3 hours until complete reaction, cooling the reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com