Sodium-ion-battery negative electrode material ferric sodium triphosphate and preparation method thereof
A sodium-ion battery, sodium iron triphosphate technology, applied in battery electrodes, secondary batteries, circuits, etc., to achieve the effects of good crystallinity, simple preparation, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Na 2 CO 3 , FeC 2 o 4 2H 2 O and NH 4 h 2 PO 4 Weigh according to the stoichiometric ratio (molar ratio) of Na:Fe:P=1.15:4:3, mix evenly, and ball mill on a planetary ball mill for 4 hours;
[0033] Then, under an argon atmosphere, pretreatment was performed at 200°C for 10 hours, and after natural cooling, a powdery product was obtained;
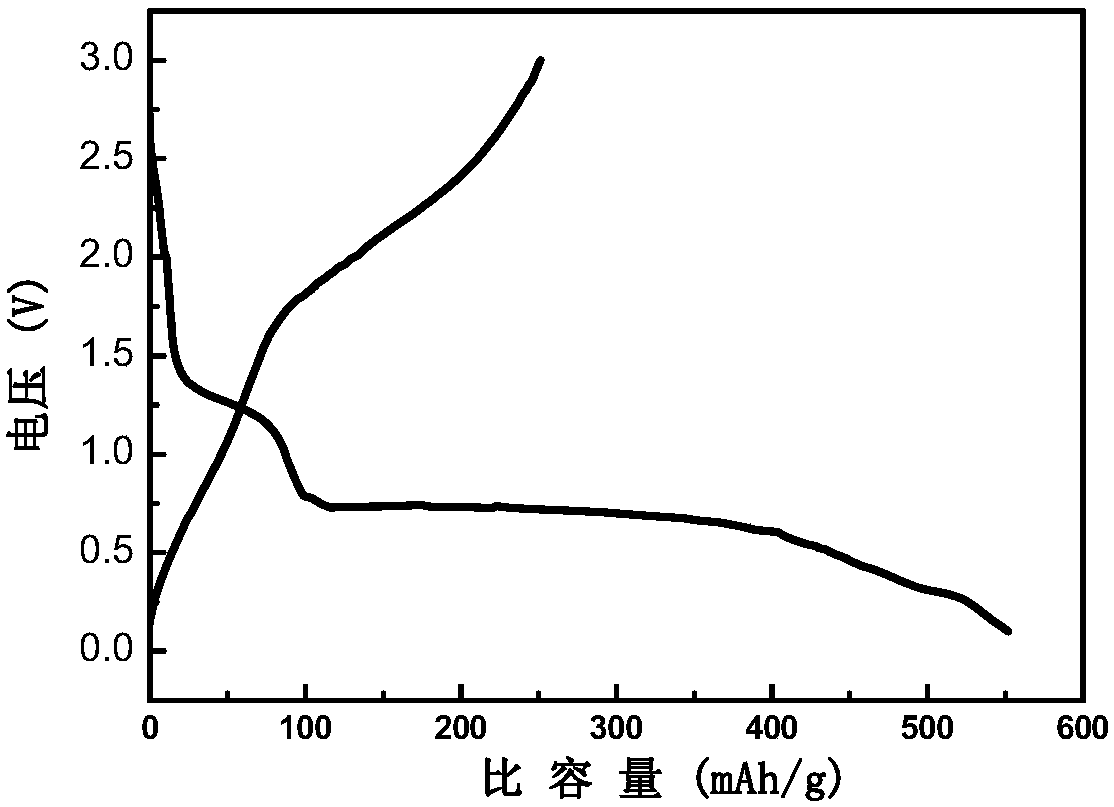
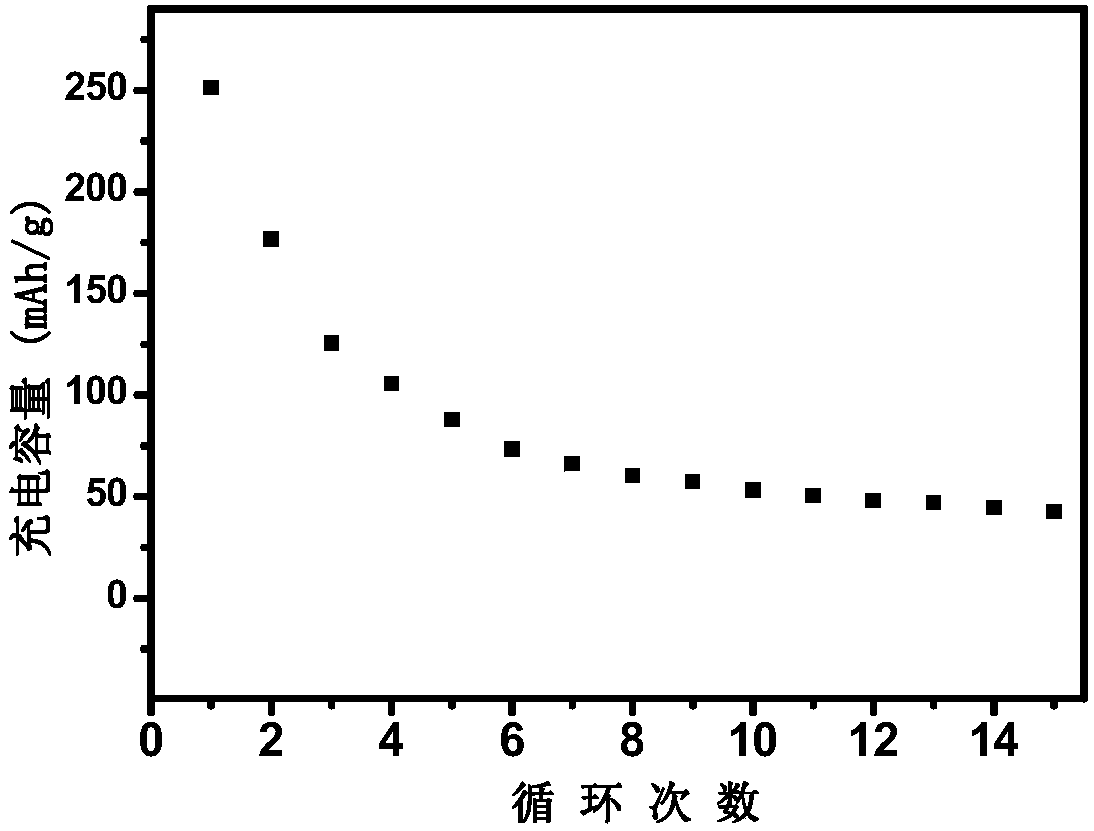
[0034] The above-mentioned powdery product was ball-milled again in a planetary ball mill for 6 h, and the 2 Under atmosphere, sintering at 850°C for 10h to obtain sodium iron triphosphate [Na 1.15 Fe 4 (PO 4 ) 3 ] Anode material.
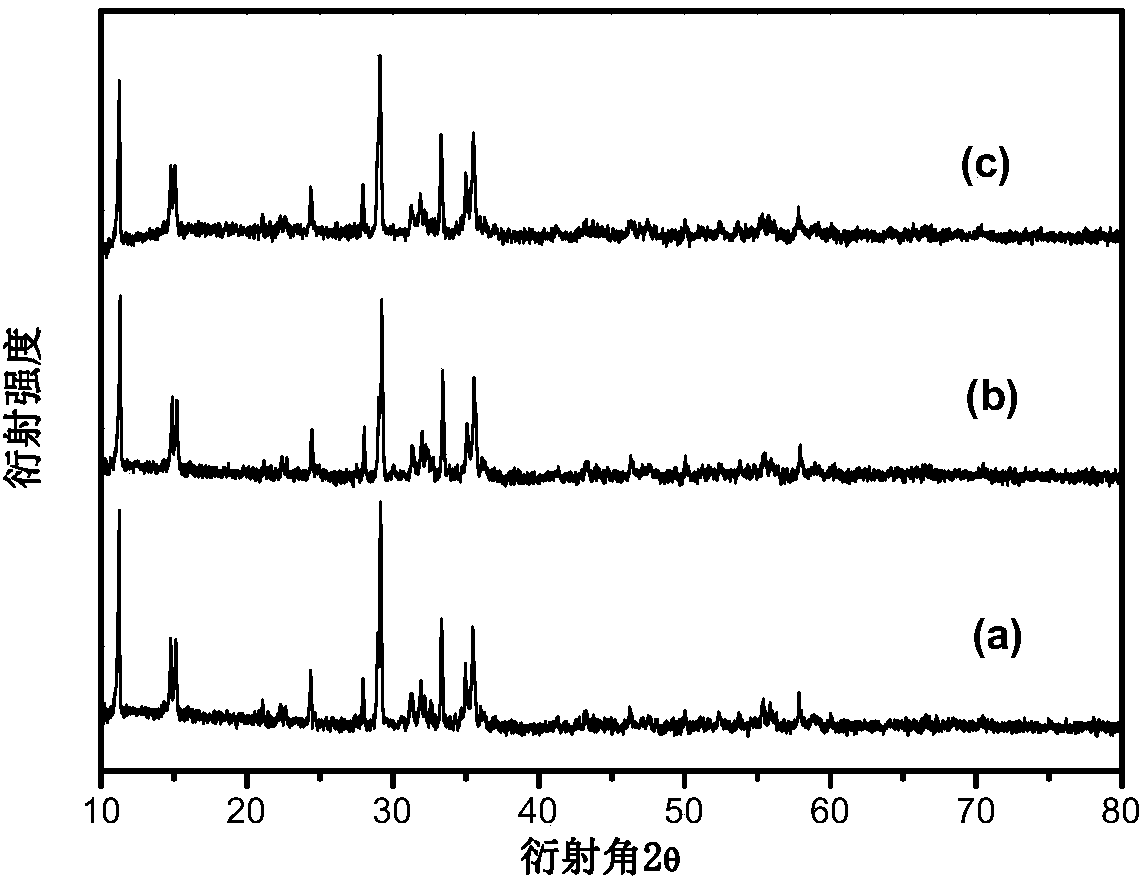
[0035] The XRD pattern of the product obtained in this embodiment is shown in figure 1 In a, it can be seen from the figure that pure-phase sodium iron triphosphate [Na 1.15 Fe 4 (PO 4 ) 3 ] Anode material. There is no impurity peak in the spectrogram, and the product has high purity.
Embodiment 2
[0037] Na 2 CO 3 , FeC 2 o 4 2H 2 O and NH 4 h 2 PO 4 Weigh according to the stoichiometric ratio (molar ratio) of Na:Fe:P=1.1:4:3, mix evenly, and ball mill on a planetary ball mill for 4 hours;
[0038] Then in Ar+5%H 2 Under air atmosphere, pretreatment was carried out at 300°C for 6 hours, and after natural cooling, a powdery product was obtained;
[0039] The pretreated product was added with 20wt% (relative to the powdered product) glucose (0.85g) as a carbon source, and ball milled again;
[0040] The above-mentioned powdery product was ball-milled again in a planetary ball mill for 6 h, under N 2 Sintering at 750°C for 20 h under air atmosphere to obtain sodium iron triphosphate [Na 1.1 Fe 4 (PO 4 ) 3 / C] anode material.
[0041] The XRD pattern of the product obtained in this embodiment is shown in figure 1 In b, it can be seen from the figure that pure phase carbon-coated sodium iron triphosphate [Na 1.1 Fe 4 (PO 4 ) 3 / C] anode material. There is...
Embodiment 3
[0043] Na 2 CO 3 , FeC 2 o 4 2H 2 O and NH 4 h 2 PO 4 Weigh according to the stoichiometric ratio (molar ratio) of Na:Fe:P=1.2:4:3, mix evenly, and ball mill on a planetary ball mill for 4 hours;
[0044] Then, under an argon atmosphere, pretreatment was carried out at 400°C for 2 hours, and after natural cooling, a powdery product was obtained;
[0045] Add 20wt% (relative to the powdered product) glucose (0.93g) to the pretreated product as a carbon source, and ball mill again;
[0046] The above-mentioned powdery product was ball-milled again in a planetary ball mill for 6 h, and the 2 Sintering at 800°C for 15 hours under air atmosphere to obtain sodium iron triphosphate [Na 1.1 Fe 4 (PO 4 ) 3 / C] anode material.
[0047] The XRD pattern of the product obtained in this embodiment is shown in figure 1 In c, it can be seen from the figure that pure phase carbon-coated sodium iron triphosphate [Na 1.2 Fe 4 (PO 4 ) 3 / C] anode material. There is no impurity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com