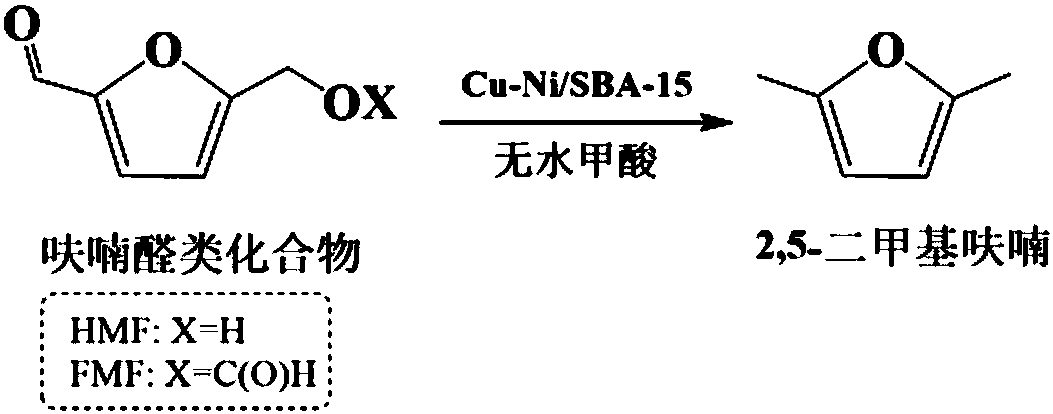
Preparation method of 2, 5-dimethylfuran
A technology of dimethylfuran and furanaldehyde, which is applied in 2 fields, can solve the problems of high transportation and storage cost, low non-precious metal conversion rate, long reaction time and the like, and achieves high selectivity, good hydrogenation effect and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 0.18g of 5-formyloxymethylfurfural to 10mL of tetrahydrofuran, then add 400μL of anhydrous formic acid and 0.10g of non-precious metal catalyst A, mix and place in a closed high-pressure reactor, replace with nitrogen 4 to 5 times to exhaust the air, 200 The mixture was heated and stirred at ℃ for 5 hours to carry out hydrodeoxygenation reaction to obtain 2,5-dimethylfuran with a yield of 40.70%.
[0025] Wherein, the mass fraction of nickel and copper accounting for carrier SBA-15 in the non-precious metal catalyst A is 48%, wherein the mass ratio of nickel and copper is 3: 1; the preparation method of catalyst A comprises the following steps: take 0.5g SBA-15, according to The mass fraction of copper and nickel was prepared by preparing nickel nitrate and copper nitrate aqueous solutions, adding them dropwise to molecular sieve SBA-15, mixing them evenly, and impregnating them in equal volumes for 12 hours; drying the impregnated products at 110°C for 10 hours to o...
Embodiment 2
[0027] Add 0.18g of 5-formyloxymethylfurfural to 10mL of tetrahydrofuran, then add 560μL of anhydrous formic acid and 0.10g of non-precious metal catalyst A, mix and place in a closed high-pressure reactor, replace with nitrogen 4 to 5 times to exhaust the air, 220 The mixture was heated and stirred at °C for hydrodeoxygenation reaction for 5 hours to obtain 2,5-dimethylfuran with a yield of 71.04%.
[0028] See Example 1 for the preparation method of non-noble metal catalyst A.
Embodiment 3
[0030] Add 0.18g of 5-formyloxymethylfurfural to 10mL of 1,4-dioxane, then add 400μL of anhydrous formic acid and 0.10g of non-precious metal catalyst A, mix them in a closed autoclave, and replace with nitrogen for 4~ The air was discharged five times, heated and stirred at 220° C. for hydrodeoxygenation reaction for 5 hours to obtain 2,5-dimethylfuran with a yield of 62.73%.
[0031] See Example 1 for the preparation method of non-noble metal catalyst A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com