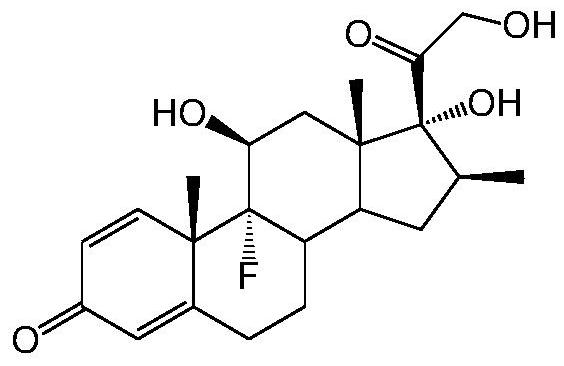
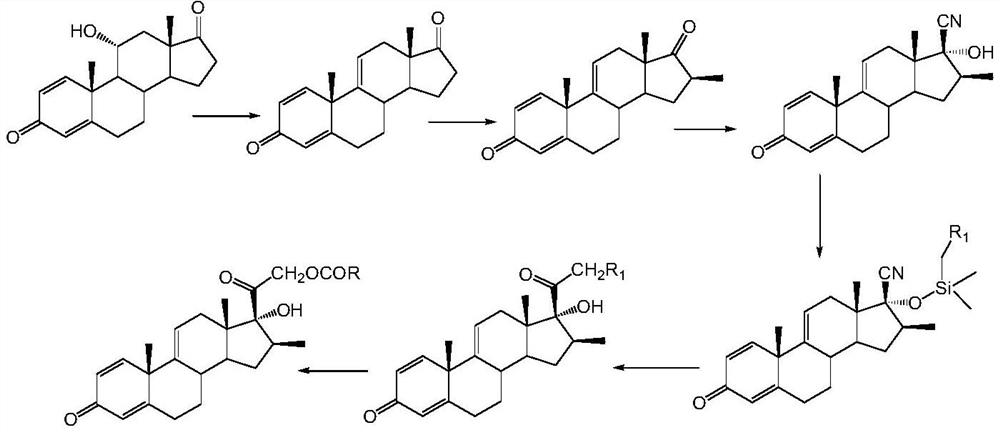
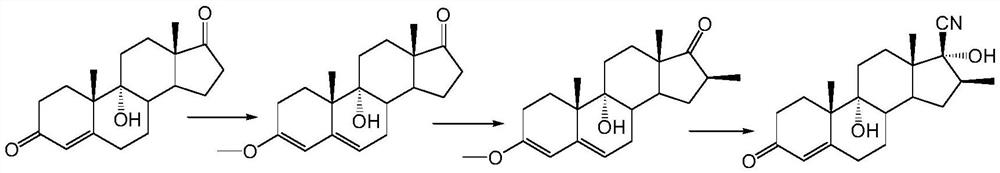
A kind of preparation method of betamethasone intermediate
A technology for betamethasone and intermediates, which is applied in the field of preparation of the side chain at the 17-position of betamethasone intermediates, can solve the problems of many side reactions in the cyanation reaction, restricting industrial production, poor product quality and the like, and achieves increased reaction steps, The effect of shortened reaction time and increased product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 17α-hydroxy-16β-methyl-17β-cyano-1,4,9(11)-trien-3-one
[0023] Silicocyanation reaction:
[0024] At room temperature, add 30g of 16β-methyl-1,4,9(11)-triene-3,17-dione to a clean and dry 250ml three-necked round-bottomed flask equipped with a thermometer and mechanical stirring, dissolve in 120ml of dichloro Add 0.5g of zinc iodide and 10.9g of trimethylsilyl cyanide to methane, keep the temperature at 25°C for 3 hours, and complete the reaction in TLC (developer: acetone / petroleum ether = 1:4), add 60ml of 5% sodium bicarbonate aqueous solution Terminate the reaction, separate the organic layer, extract the aqueous phase with 30 ml of dichloromethane, combine the organic phases and wash with 100 ml of water, dry the organic phase with anhydrous sodium sulfate, rise to 40 ° C and concentrate under reduced pressure to a small volume, and bring to Without dichloromethane, it was stirred and filtered with suction, and the solid was dried at 40°...
Embodiment 2
[0029] Example 2: Preparation of 9,17α-hydroxy-16β-methyl-17β-cyano-4,9(11)-dien-3-one
[0030] Silicocyanation reaction:
[0031] At room temperature, add 54g of 9α-hydroxyl-16β-methyl-4,9(11)-diene-37-dione to a 500ml three-necked round-bottomed flask equipped with a thermometer and mechanically stirred, which is clean and dry, and dissolve in 270ml of dichloro In methane, add 1.1g cuprous iodide, 19.1g trimethylsilyl cyanide, 30 ℃ heat preservation reaction for 2h, TLC control reaction is complete (developer: acetone / petroleum ether = 1:4), add 50ml 10% bicarbonate The reaction was terminated by sodium aqueous solution, the organic layer was separated, the aqueous phase was extracted with 40ml of dichloromethane, the organic phase was combined and washed with 100ml of water, the organic phase was dried with anhydrous sodium sulfate, raised to 40°C and concentrated under reduced pressure to a small volume, and then washed with petroleum ether Take until there is no dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com