Preparation method of high-activity methane halogen oxidation catalyst
A methane halogen oxidation and catalyst technology, which is applied in the field of preparation of highly active methane halogen oxidation catalysts, can solve problems such as low yield of methyl halides and reduced selectivity of methyl halides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
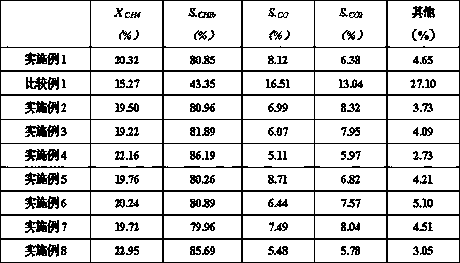
Examples
Embodiment 1
[0028] Preparation of modified alumina: using equal volume impregnation method on alumina (commercially available, the properties are as follows: specific surface 335m 2 / g, the pore volume is 0.86ml / g) impregnated with gallium nitrate and nickel nitrate aqueous solution, the molar concentration of gallium ions in the solution is 1 mol / L, and the molar concentration of nickel metal ions is 0.5 mol / L, after impregnation, it is dried and roasted. The drying time is 1h, and the drying temperature is 100°C; the calcination time is 2h, and the temperature is 900°C;
[0029] Preparation of zinc-loaded alumina: impregnating zinc nitrate solution on the modified alumina by equal volume impregnation, drying and roasting after impregnation, the drying time is 2 hours, and the drying temperature is 130°C; the roasting time is 4 hours, the temperature 400°C, the zinc-loaded alumina is spherical, and the equivalent diameter of the zinc-loaded alumina is 2mm;
[0030] Prepare aluminum hydr...
Embodiment 2
[0035] Preparation of modified alumina: using equal volume impregnation method on alumina (commercially available, the properties are as follows: specific surface 335m 2 / g, pore volume is 0.86ml / g) impregnated with gallium nitrate and nickel nitrate aqueous solution, the molar concentration of gallium ions in the solution is 3mol / L, and the molar concentration of nickel metal ions is 1.5mol / L, after impregnation, it is dried and roasted. The drying time is 2h, and the drying temperature is 100°C; the roasting time is 8h, and the temperature is 700°C;
[0036] Preparation of zinc-loaded alumina: impregnate the modified alumina with a zinc sulfate solution using an equal-volume impregnation method, dry and roast after impregnation, the drying time is 3 hours, and the drying temperature is 120 ° C; the roasting time is 5 hours, the temperature is 450°C, the zinc-loaded alumina is spherical, and the equivalent diameter of the zinc-loaded alumina is 2mm;
[0037] Prepare aluminum...
Embodiment 3
[0042] Preparation of modified alumina: using equal volume impregnation method on alumina (commercially available, the properties are as follows: specific surface 335m 2 / g, pore volume is 0.86ml / g) impregnated gallium nitrate, nickel nitrate aqueous solution, the molar concentration of gallium ion in the solution is 2mol / L, the molar concentration of nickel metal ion is 1mol / L, after impregnating, carry out drying, roasting, described The drying time is 0.5h, and the drying temperature is 130°C; the calcination time is 5h, and the temperature is 800°C;
[0043] Preparation of zinc-loaded alumina: impregnating zinc bromide solution on the modified alumina by an equal volume impregnation method, drying and roasting after impregnation, the drying time is 4 hours, and the drying temperature is 100 ° C; the roasting time is 4 hours, The temperature is 500°C, the zinc-loaded alumina is spherical, and the equivalent diameter of the zinc-loaded alumina is 3mm;
[0044] Prepare aluminu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com