Nitrogen-containing macrocyclic compound as well as preparation method and medicinal composition and application thereof
A technology for macrocycles and compounds, applied in the field of nitrogen-containing macrocycles, can solve the problems of weak binding between bromodomains and acetylated proteins, and the influence of druggability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
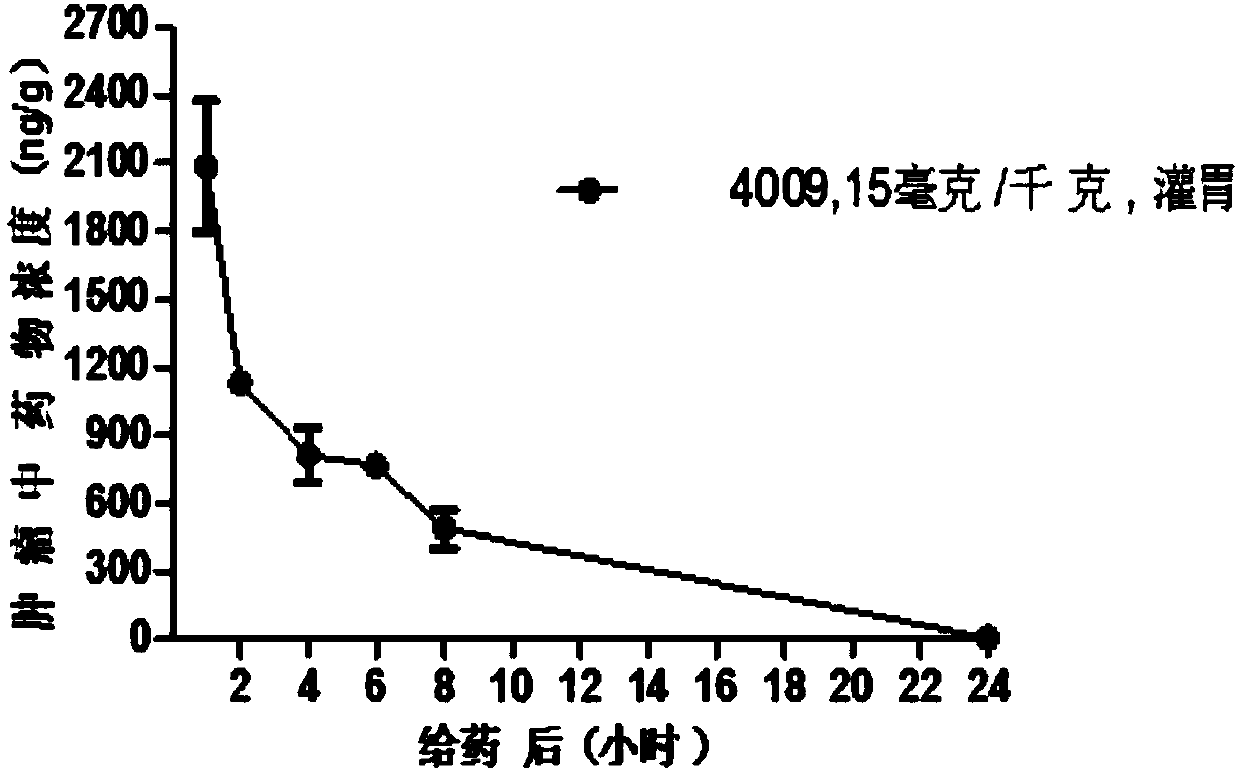
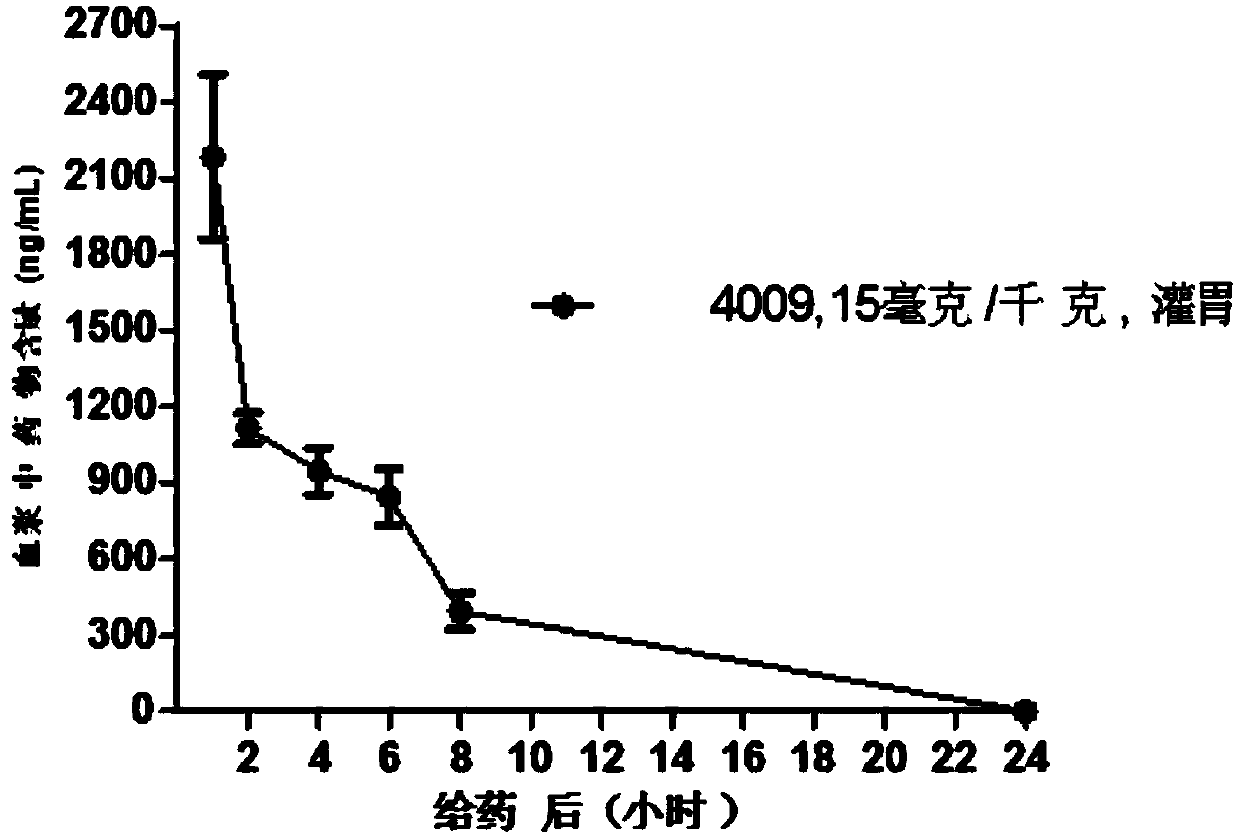
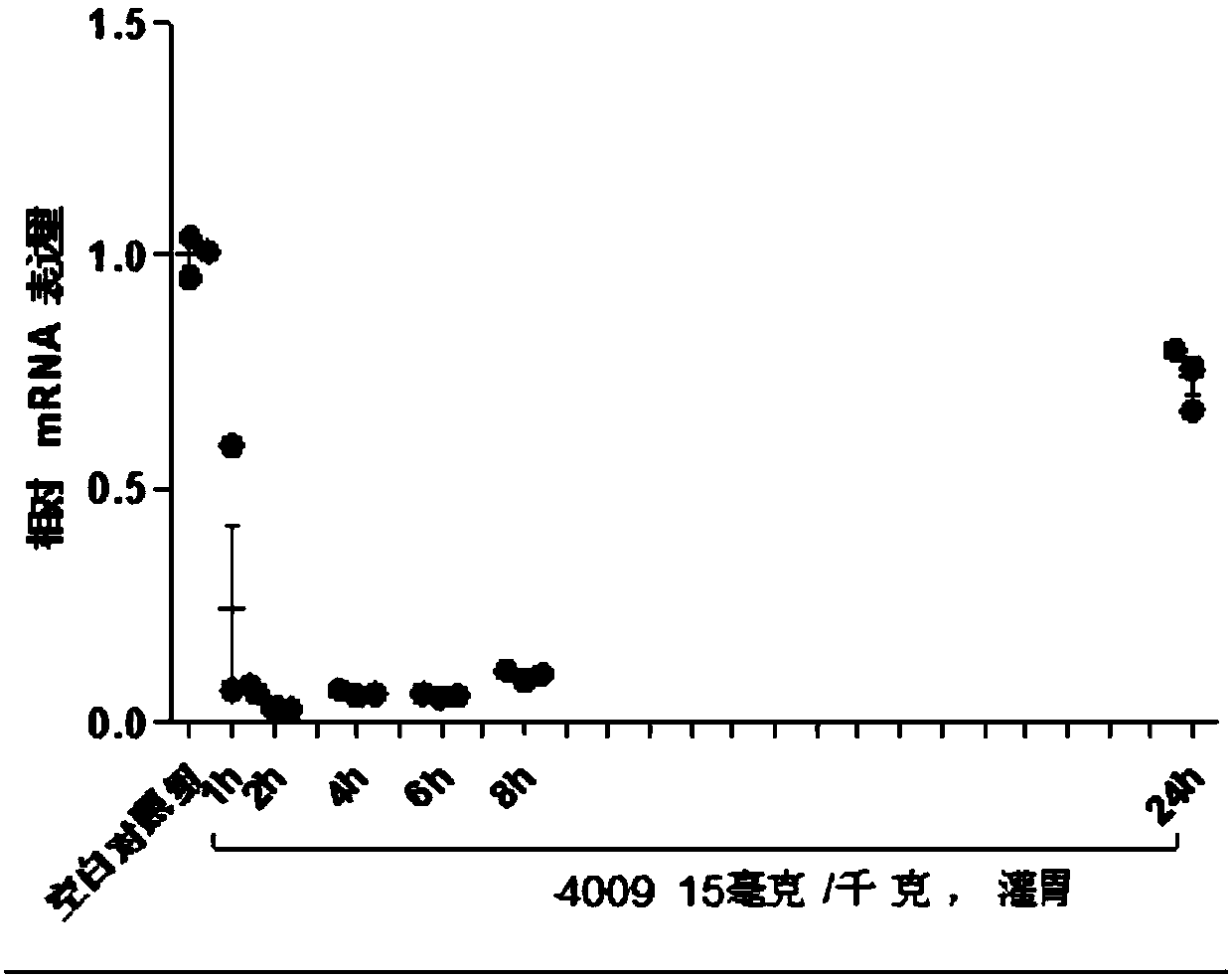
Image
Examples
Embodiment 4003
[0679] Example 4003: 6-(4-fluorophenyl)-2-methyl-9-(methylsulfonemethyl)-5-oxo-2,4,5,6-tetrahydrobenzo[b]pyrrole And[3,4-d]azepine-3-carbonitrile
[0680]
[0681] Step 1: 1-(Methylsulfonemethyl)-4-nitrobenzene
[0682]
[0683] 4-Nitrobenzyl bromide (10.0 g, 46.4 mmol) was dissolved in N,N-dimethylformamide (25 mL), then sodium methylsulfinate (7.10 g, 69.6 mmol) was added. The reaction solution was stirred at 65°C for 1 hour, cooled to room temperature, diluted with water, continued to stir for 10 minutes, filtered and dried to give the title compound (9.7 g, 97%) as a white solid.
[0684] Step 2: 4-(Methylsulfonemethyl)aniline
[0685]
[0686] Dissolve 1-(methylsulfonemethyl)-4-nitrobenzene (9.7g, 45.1mmol), iron powder (12.6g, 225.6mmol) and ammonium chloride (28.4g, 451.1mmol) in ethanol (120mL) and water (60 mL), and stirred at 90°C for 2 hours. The solids were filtered off and the filtrate was concentrated. The residue was redissolved in ethyl acetate (5...
Embodiment 4001
[0716] Example 4001: 6-(4-Fluorophenyl)-2-methyl-9-(methylsulfonemethyl)-5-oxo-2,4,5,6-tetrahydrobenzo[b]pyrrole And[3,4-d]azepine-3-carboxamide
[0717]
[0718] 6-(4-fluorophenyl)-2-methyl-9-(methylsulfonemethyl)-5-oxo 2,4,5,6-tetrahydrobenzo[b]pyrrolo[3, 4-d] Azepine-3-carbonitrile (42mg, 0.099mmol) and potassium carbonate (41mg, 0.297mmol) were dissolved in dimethylsulfoxide (2mL), then 30% aqueous hydrogen peroxide (0.5mL) was added , and the reaction solution was stirred at room temperature for 3 hours. The reaction was quenched with water and extracted with ethyl acetate, the ethyl acetate layer was separated, washed with water and saturated brine, dried (anhydrous sodium sulfate), filtered and concentrated in vacuo, the residue was separated and purified by reverse phase prep-HPLC, The title compound (17.7 mg, 40%) was obtained as a white solid. LCMS(ESI)[M+H] + = 442.1; 1 H NMR (400MHz, DMSO-d 6 )δ7.54(s,1H),7.46-7.25(m,3H),7.23-7.13(m,3H),7.10-7.01(m,2H),6.9...
Embodiment 4004
[0719] Example 4004: 6-(4-fluorophenyl)-2-methyl-9-(methylsulfonemethyl)-2,4,5,6-tetrahydrobenzo[b]pyrrolo[3,4 -d]azepine-3-carbonitrile
[0720]
[0721] 6-(4-fluorophenyl)-2-methyl-9-(methylsulfonemethyl)-5-oxo 2,4,5,6-tetrahydrobenzo[b]pyrrolo[3, 4-d] Azepine-3-carbonitrile (50mg, 0.118mmol) was dissolved in tetrahydrofuran (5mL), and borane (1M solution in tetrahydrofuran, 0.5mL) was added at 0°C under nitrogen protection, and then warmed to room temperature Stir for 1 hour. Methanol (5 mL) was added to the reaction solution, the reaction solution was concentrated, and the residue was separated and purified by preparative-HPLC to obtain the title compound (14.6 mg, 30%) as a white solid. LCMS(ESI)[M+H] + = 410.1; 1 H NMR (400MHz, DMSO-d 6 )δ7.84(s,1H),7.62(s,1H),7.25-7.19(m,2H),6.98(t,J=7.2Hz,3H),6.76-6.70(m,2H),4.48(s ,2H),3.88(t,J=4.4Hz,2H),3.75(s,3H),3.01(t,J=4.4Hz,2H),2.96(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com