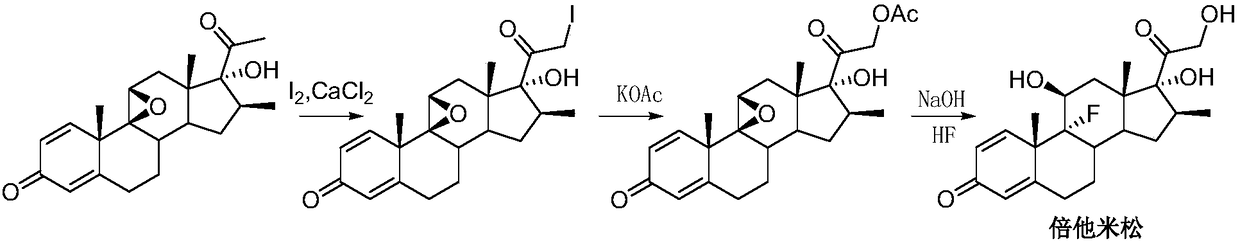
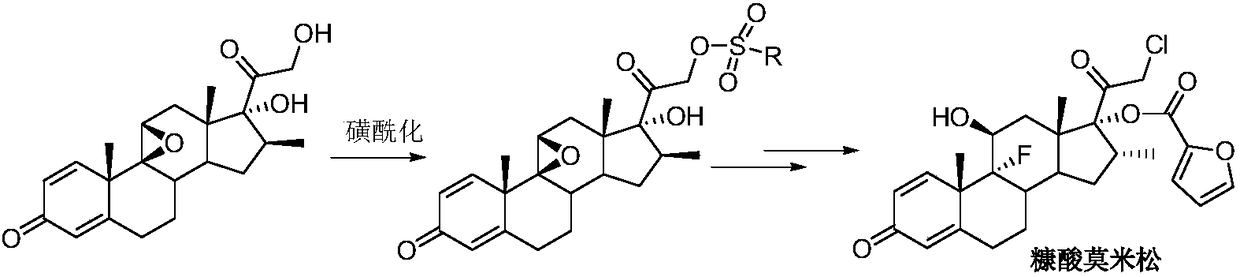
Preparation method for 21-halogenated steroid
A technology for halogenated steroids and compounds, which is applied in the field of preparation of 21-halogenated steroids and can solve the problems of short steps and complicated reaction process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
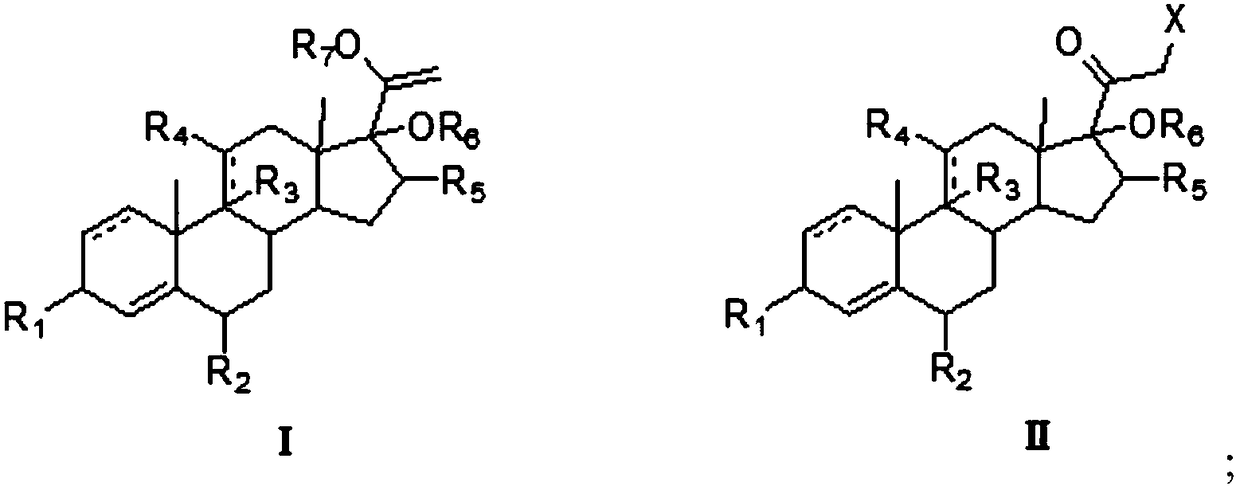
[0032] The preparation method of the 21-halogenated steroid compound of one embodiment, comprises the following steps:
[0033] The compound of formula I is used as a raw material, and the 21-halogenated reaction with a halogenating reagent is carried out in a mixed solvent of organic solvent and water, and then hydrolyzed under acidic conditions to obtain a 21-halogenated steroid compound of formula II.
[0034] The structural formulas of the 21-halogenated steroid compound of formula I and formula II are as follows:
[0035]
[0036] Wherein, the dotted line represents a single bond or a double bond;
[0037] R 1 is carbonyl or OH;
[0038] R 2 for H, CH 3 , Cl or F;
[0039] R 3 Is H, F, Cl, OH or no group, R 4 is carbonyl, OH or H; or, R 3 with R 4 For the epoxy group;
[0040] R 5 H, α-CH 3 or β-CH 3 ;
[0041] R 6 is H, Si(CH 3 ) 3 、COCH 3 、COCH 2 CH 3 ;
[0042] R 7 for Si(CH 3 ) 3 、COCH 3 、COCH 2 CH 3 、CH 3 or CH 2 CH 3 ;
[0043] X is...
Embodiment 1
[0066]
[0067] In a three-necked flask with a thermometer and a stirring magnet, add 100g (0.364mol) of compound Ia, 5mL (0.036mol) of triethylamine, 500mL of acetone and 100mL of water, stir evenly, and cool the system to 0-5°C. (0.300mol) NCS was slowly added to the reaction flask in batches, and the temperature was kept at 0-5°C during the process. NCS was added in 5 batches, and added once every 20 minutes. After the addition was completed, continue to keep stirring for 1-2 hours. TLC detects that the reaction raw materials disappear completely (benzene:acetone=6:1), stop the reaction, add dropwise 100mL of 20wt% sodium sulfite solution to quench the reaction, add 30mL of 36wt% concentrated hydrochloric acid, adjust the pH to about 2, and raise the temperature of the system to 25-30 The reaction was continued at ℃ for 1-3 hours, TLC detected that the hydrolysis was complete, and 20% NaOH solution was added dropwise to adjust the system to neutrality. Concentrate the ac...
Embodiment 2
[0069]
[0070] In a three-neck flask with a thermometer and a stirring magnet, add 100g (0.314mol) of compound Ib, 500mL of acetone, 100mL of water and 5g (0.047mol) of sodium carbonate and stir evenly. The system is cooled to 5-10°C, and 40g (0.300 mol) NCS was slowly added to the reaction bottle in batches, and the temperature was kept at 5-10°C during the process. NCS was added in 5 batches, and added once every 20 minutes. After the addition was completed, continue to keep stirring for 1-2 hours. TLC detects that the reaction raw materials disappear completely (benzene:acetone=6:1), stop the reaction, add 20g of sodium sulfite solid to quench the reaction and stir for 30min, add 30mL of 36wt% concentrated hydrochloric acid dropwise, adjust the pH of the system to 1, and heat up to 30-40°C The reaction was continued for 1-3 hours, and the hydrolysis was detected by TLC, and 20% NaOH solution was added dropwise to adjust the system to neutrality. Concentrate the acetone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com