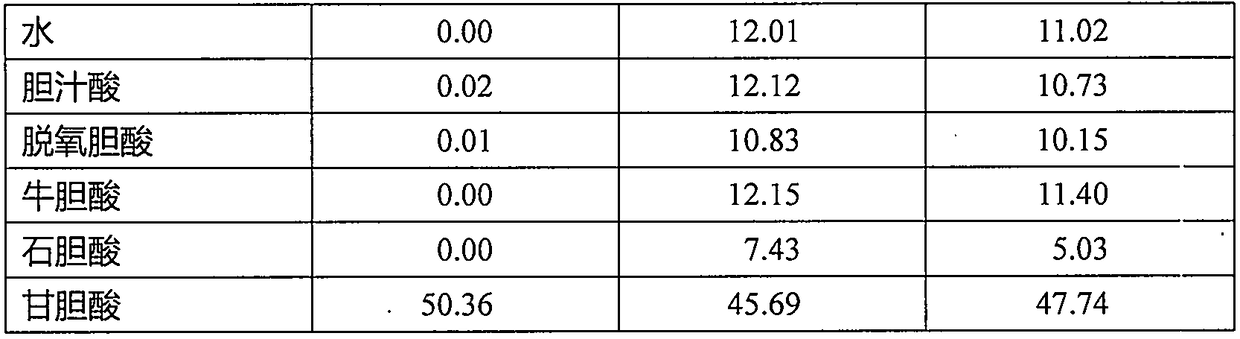
Enzymatic colorimetric method and reagent for measuring concentration of glycocholic acid
A technology of glycocholic acid and detection method, which is applied to the measurement of color/spectral characteristics, etc., can solve the problems of poor anti-interference performance and low accuracy, and achieve the effects of low cost, high accuracy, and simple and easy-to-use methodology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of Reagent 1:
[0044] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 1:
[0045] 100mM / L Tris buffer pH 7.90
[0046] 50mM / L sodium chloride
[0047] 1mM / L EDTA
[0048] 20mM / L Mannitol
[0049] 0.02% Proclin-300
[0050] 0.1% Tween-20
[0051] 0.2% bovine serum albumin
[0052] 4MU / L catalase
[0053] 5KU / L ascorbate oxidase
[0054] 20KU / L glycine oxidase
[0055] 0.3mM / L 4-AA
[0056] Preparation of Reagent 2:
[0057] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 2:
[0058] 100mM / L Tris buffer pH7.50
[0060] 100mmol / L Mannitol
[0061] 50mM / L sodium chloride
[0063] 1mM / L EDTA
[0064] 0.1% Tween-20
[0065] 0.2% bovine serum albumin
[0066] 2KU / L peroxidase
[0067] 50KU / L glycocholic acid hydrolase
[0068] 10mM / L TBHBA
Embodiment 2
[0070] Preparation of Reagent 1:
[0071] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 1:
[0072] 100mM / L Tris buffer pH 7.90
[0073] 100mM / L sodium chloride
[0074] 5mM / L EDTA
[0075] 100mM / L Mannitol
[0076] 0.02% Proclin-300
[0077] 0.3% Tween-20
[0078] 0.5% bovine serum albumin
[0079] 5MU / L catalase
[0080] 5KU / L ascorbate oxidase
[0081] 50KU / L glycine oxidase
[0082] 0.2mM / L SMBTH
[0083] Preparation of Reagent 2:
[0084] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 2:
[0085] 100mM / L Tris buffer pH7.50
[0086] 0.15% sodium azide
[0087] 100mmol / L Mannitol
[0088] 50mM / L sodium chloride
[0090] 2mM / L EDTA
[0091] 0.3% Tween-20
[0092] 0.2% bovine serum albumin
[0093] 2KU / L peroxidase
[0094] 100KU / L Glycocholic Acid Hydrolase
[0095] 2mM / L ALPS
Embodiment 3
[0097] Preparation of Reagent 1:
[0098] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 1:
[0099] 50mM / L 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid buffer pH 8.00
[0100] 50mM / L sodium chloride
[0101] 2mM / L EDTA
[0102] 100mM / L Mannitol
[0103] 0.02% Proclin-300
[0104] 0.1% Triton X-100
[0105] 0.2% bovine serum albumin
[0106] 5MU / L catalase
[0107] 5KU / L ascorbate oxidase
[0108] 5KU / L glycine oxidase
[0109] 0.3mM / L CCAP
[0110] Preparation of Reagent 2:
[0111] Dissolve the following substances in pure water in sequence according to each concentration to prepare reagent 2:
[0112] 100mM / L 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid buffer pH8.00
[0113] 0.15% sodium azide
[0114] 100mmol / L Mannitol
[0115] 50mM / L sodium chloride
[0117] 1mM / L EDTA
[0118] 0.1% Triton X-100
[0119] 0.2% bovine serum albumin
[0120] 2KU / L pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com