Spiroindene compound and its preparation and application
A compound, spiroindene technology, applied in the field of organic light-emitting materials, can solve the problems that affect the development of full-color OLED white light devices, low color life, poor thermal stability, etc., to achieve improved shape stability, high luminous quantum efficiency, and thermal stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
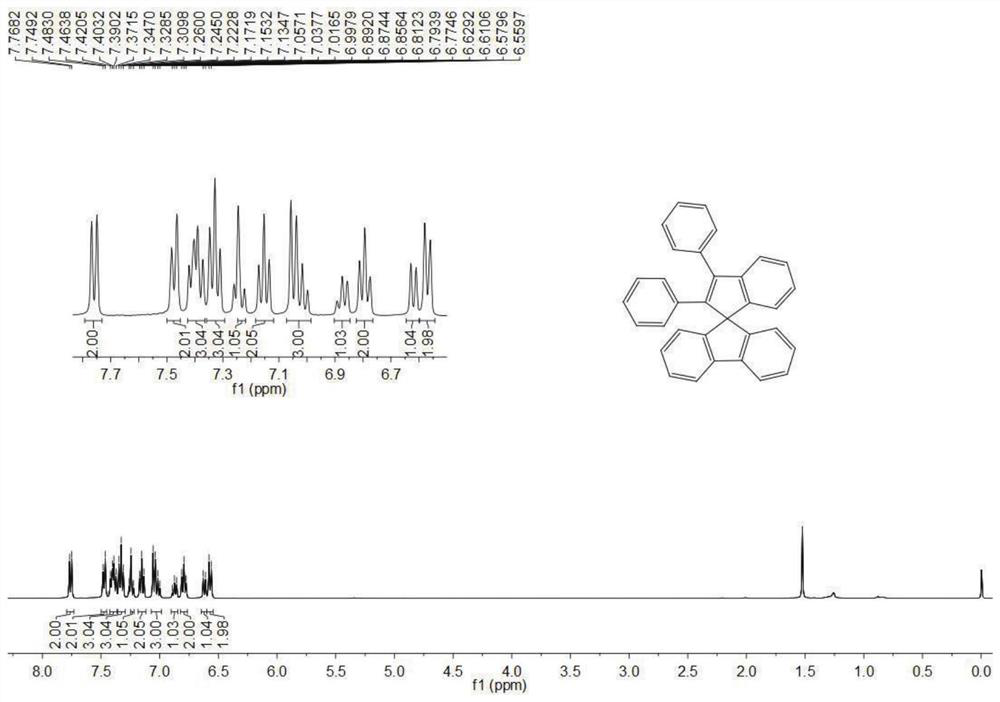
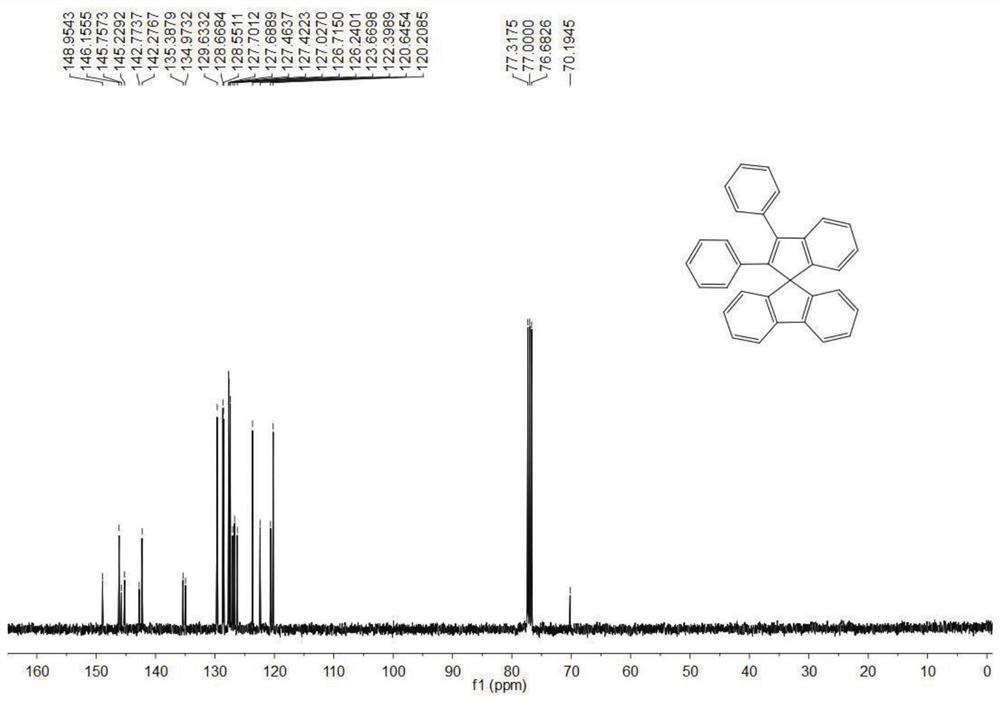
[0066] Synthesis of Compound 1-A
[0067] (1) Synthesis of 9-(1,2,2-triphenylvinyl)-9H-fluorene-9-ol
[0068] Under the protection of an inert gas, add 2g (0.006mol) triphenylbromide to a 100mL Schuck bottle, inject 120mL of anhydrous tetrahydrofuran solvent, stir for 15min after dissolving, and then inject 2.8mL (0.007mol, 2.5mol / L) The hexane solution of butyllithium was stirred and reacted for 1 hour, and then 1.26 g (0.007 mol) of 9H-fluoren-9-one was added, and the reaction was continued for 8 hours. After the reaction was completed, 0.1 mL of water was injected to quench the reaction, the solvent was spin-dried and purified by chromatographic chromatography for the next reaction.
[0069] (2) Preparation of structural formula 1-A compound
[0070] Under the protection of an inert gas, add 0.0011 mol of the above crude product to a 100 mL two-necked flask, add 30 mL of dichloromethane solvent and 0.0056 mol of stannous chloride. React at room temperature for three hour...
Embodiment 2
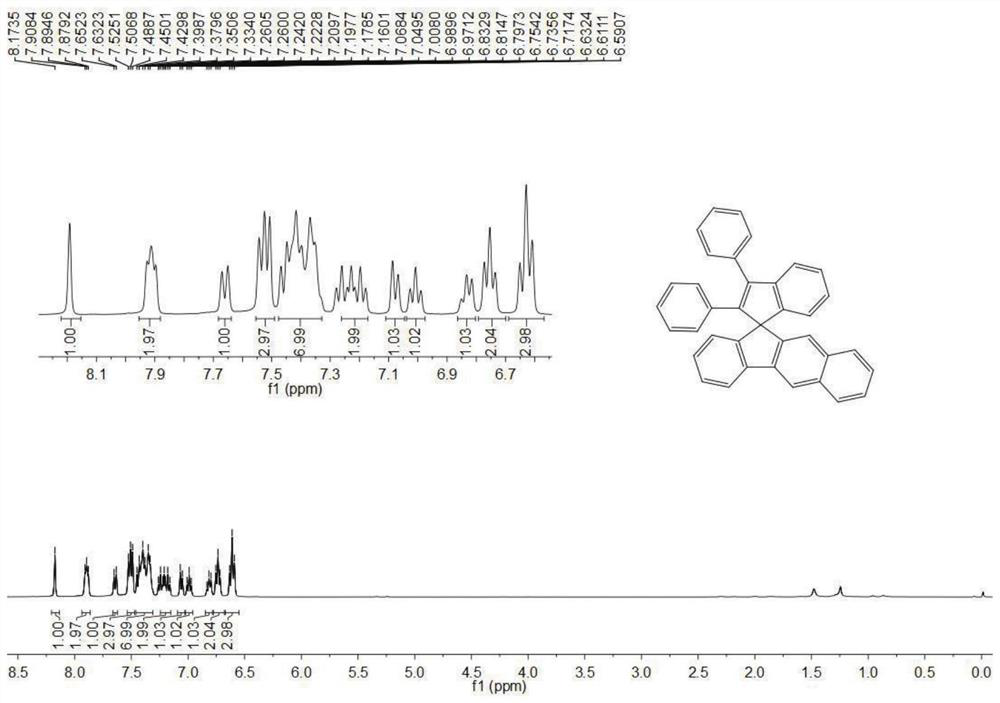
[0072] Synthesis of Compound 1-B
[0073] The specific steps are the same as in Example 1, only the raw material 9H-fluoren-9-one in (1) is changed to 11H-benzo[b]fluoren-11-one
Embodiment 3
[0075] Synthesis of Compound 1-C
[0076] The specific steps are the same as in Example 1, except that the raw material 9H-fluoren-9-one in (1) is changed to 11H-benzo[a]fluoren-11-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com