Preparation method of isovanillin
A technology of isovanillin and ethyl vanillin, which is applied in the field of preparation of isovanillin, can solve the problems of process by-products polluting the environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation method of isovanillin, comprising the steps of:
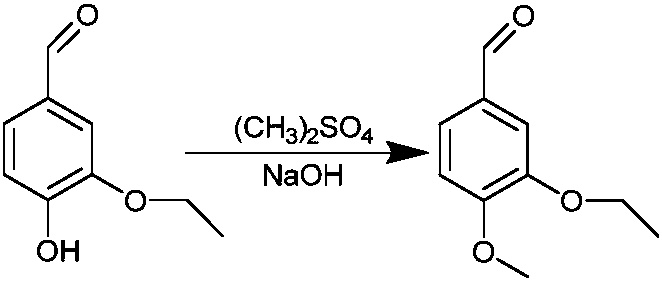
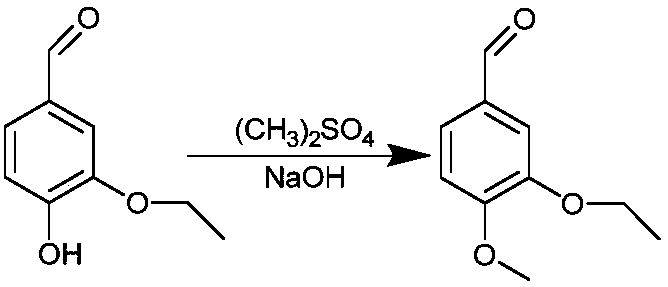
[0051] (1) Dimethyl sulfate and ethyl vanillin solution were reacted for 6 hours in an alkaline environment at a temperature of 20° C., filtered, and the filter residue was taken to obtain an intermediate.
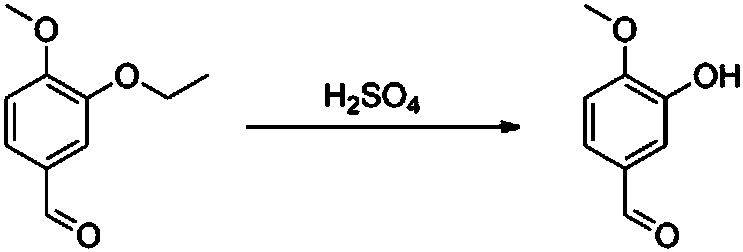
[0052] (2) The intermediate and concentrated sulfuric acid were reacted for 5 hours in an inert gas atmosphere at a temperature of 50°C and under negative pressure conditions.
Embodiment 2
[0054] A preparation method of isovanillin, comprising the steps of:
[0055] (1) Adding ethyl vanillin to a potassium hydroxide solution with a pH of 12 to obtain an ethyl vanillin solution.
[0056] (2) Dimethyl sulfate and ethyl vanillin solution were reacted in an alkaline environment at a temperature of 30° C. for 6 hours, filtered, washed and dried to obtain an intermediate. Wherein, the mass ratio of ethyl vanillin to dimethyl sulfate is 1:1.
[0057] (3) Put the intermediate into an enamel reaction kettle, and add concentrated sulfuric acid with a mass concentration of 92% after passing through nitrogen, and react at a temperature of 60°C and a pressure of -92Kpa for 3 hours to obtain crude isovanillin. Among them, the mass ratio of the intermediate to the concentrated sulfuric acid is 1:2.
[0058] (4), transfer the reacted isovanillin crude product into water to dilute and then extract with ethyl acetate to obtain isovanillin, wherein the mass ratio of concentrated...
Embodiment 3
[0060] A preparation method of isovanillin, comprising the steps of:
[0061] (1) Adding ethyl vanillin to sodium hydroxide solution with a mass concentration of 8% to obtain ethyl vanillin solution.
[0062] (2) Dimethyl sulfate and ethyl vanillin solution were reacted in an alkaline environment at a temperature of 5° C. for 4 hours, filtered, washed and dried to obtain an intermediate. Among them, the mass ratio of ethyl vanillin to dimethyl sulfate is 1:0.8.
[0063] (3) Add the intermediate to an enamel reaction kettle, and add concentrated sulfuric acid with a mass concentration of 90% after passing through nitrogen, and react at a temperature of 40°C and a pressure of -90Kpa for 2 hours to obtain crude isovanillin. Among them, the mass ratio of the intermediate to the concentrated sulfuric acid is 1:1.5.
[0064] (4) Transfer the reacted crude isovanillin into ice water for dilution, extract with ethyl acetate, and distill under reduced pressure to obtain pure isovanil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com