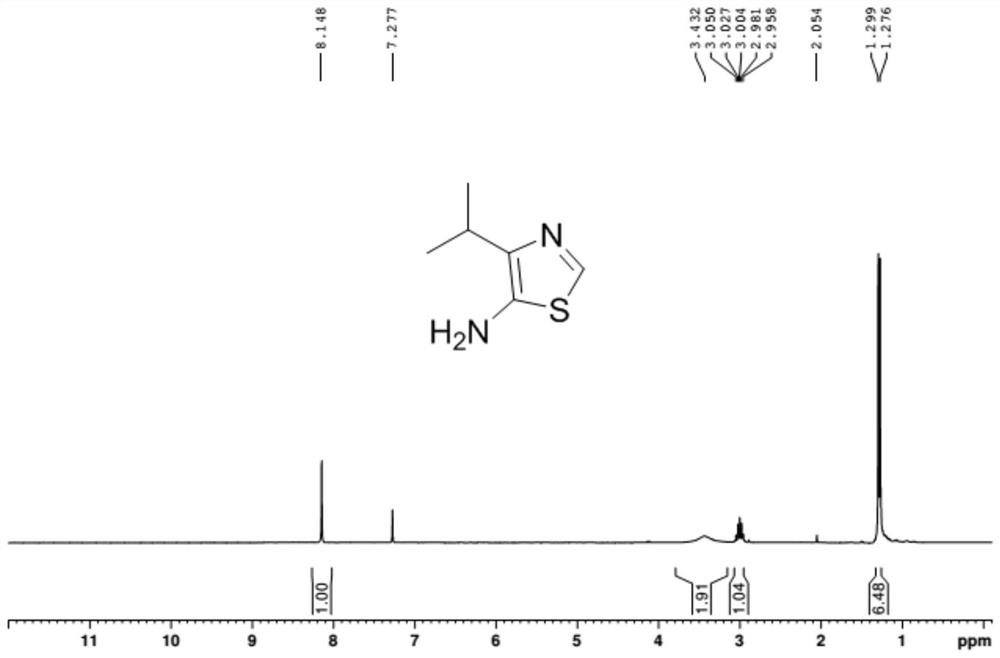
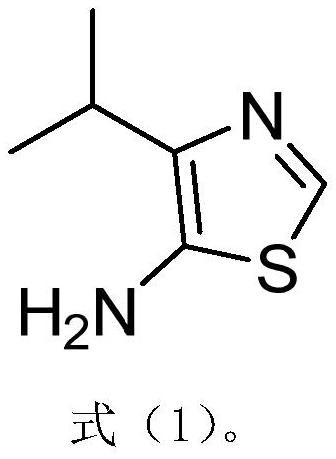
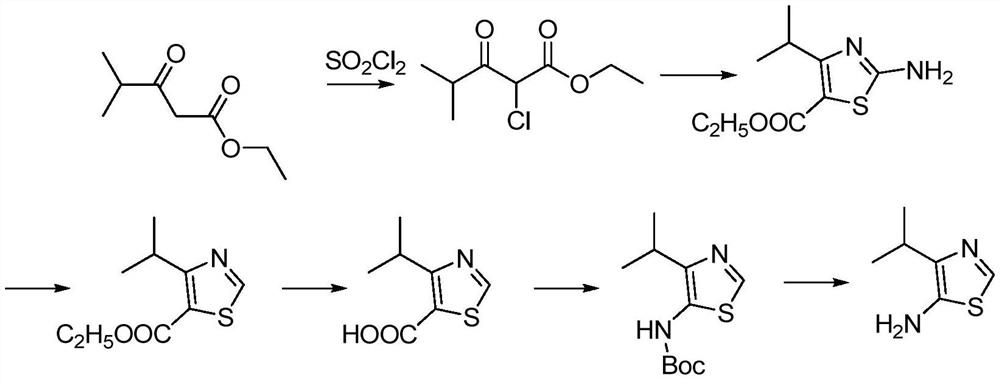
A kind of synthetic method of the amine compound containing sulfur nitrogen heterocycle of medicine intermediate
A technology of mixtures and systems, applied in the direction of organic chemistry, can solve problems such as inability to promote, and achieve the effects of saving synthesis steps, high yield, and saving raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] S1. Raw material preparation: 4 grams of ethyl 4-methyl-3-oxopentanoate, 40 ml of dichloromethane, and 4 grams of sulfonyl chloride. Select a 2L four-necked bottle with a water separator, and first put 4 grams of Dissolve ethyl 4-methyl-3-oxopentanoate in 40ml of dichloromethane, then put the mixture into a four-neck flask, stir and lower the temperature to below 0°C, add 4 grams of sulfuric acid dropwise at a very slow speed Acyl chlorides, used for no more than 30 minutes, then adjusted to pH = 7.5 with saturated aqueous sodium bicarbonate solution, then separated and extracted with 400ml of dichloromethane, washed with saturated brine, dried, and removed from the solvent to obtain the product of step S1 2-chloro-4- Ethyl Methyl-3-oxopentanoate.
[0022] S2. Take another 2L four-neck bottle, put 100ml of ethanol in it, and slowly add 12 grams of thiourea under stirring, keep the system at room temperature, and dropwise add the product of the aforementioned S1 step 2-c...
Embodiment 2
[0028] S1. Prepare 5 grams of ethyl 4-methyl-3-oxopentanoate, 50 ml of dichloromethane, and 5 grams of sulfonyl chloride as raw materials. Select a 2L four-necked bottle with a water separator, and first put 5 grams of Dissolve ethyl 4-methyl-3-oxopentanoate in 50ml of dichloromethane, then put the mixture into a four-necked flask, stir and lower the temperature to below 0°C, and drop 5 grams of sulfur at a very slow speed Acyl chloride, used for no more than 30 minutes, then adjusted to pH=8 with saturated aqueous sodium bicarbonate solution, then separated and extracted with 500ml of dichloromethane, washed with saturated brine, dried, and removed from the solvent to obtain the product of step S1 2-chloro-4- Ethyl Methyl-3-oxopentanoate.
[0029] S2. Take another 2L four-neck bottle, put 150ml of ethanol in it, and slowly add 13.7g of thiourea under stirring, keep the system at room temperature, and dropwise add the product 2-chloro-4-methyl-3-oxo of the aforementioned S1 st...
Embodiment 3
[0035] S1. Raw materials prepare 6 grams of ethyl 4-methyl-3-oxopentanoate, 60 ml of dichloromethane, and 6 grams of sulfonyl chloride. Select a 2L four-necked bottle with a water separator, and first put 6 grams of Dissolve ethyl 4-methyl-3-oxopentanoate in 60ml of dichloromethane, then put the mixture into a four-neck flask, stir and lower the temperature to below 0°C, add 6 grams of sulfur Acyl chlorides, used for no more than 30 minutes, then adjusted to pH=8.5 with saturated aqueous sodium bicarbonate solution, then separated and extracted with 600ml of dichloromethane, washed with saturated brine, dried, and removed from the solvent to obtain the product of step S1 2-chloro-4- Ethyl Methyl-3-oxopentanoate.
[0036] S2. Take another 2L four-neck bottle, put 200ml of ethanol in it, and slowly add 15 grams of thiourea under stirring, keep the system at room temperature, and dropwise add the product of the aforementioned S1 step 2-chloro-4-methyl-3-oxo 25 grams of ethyl val...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com