Cyclodextrin-base nitric oxide donor and method for preparing same
A technology of nitric oxide and cyclodextrin, which is applied in cardiovascular system diseases, antineoplastic drugs, drug combinations, etc., can solve the problems of short half-life and poor stability, and achieve long half-life, good stability, and rapid endothelialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
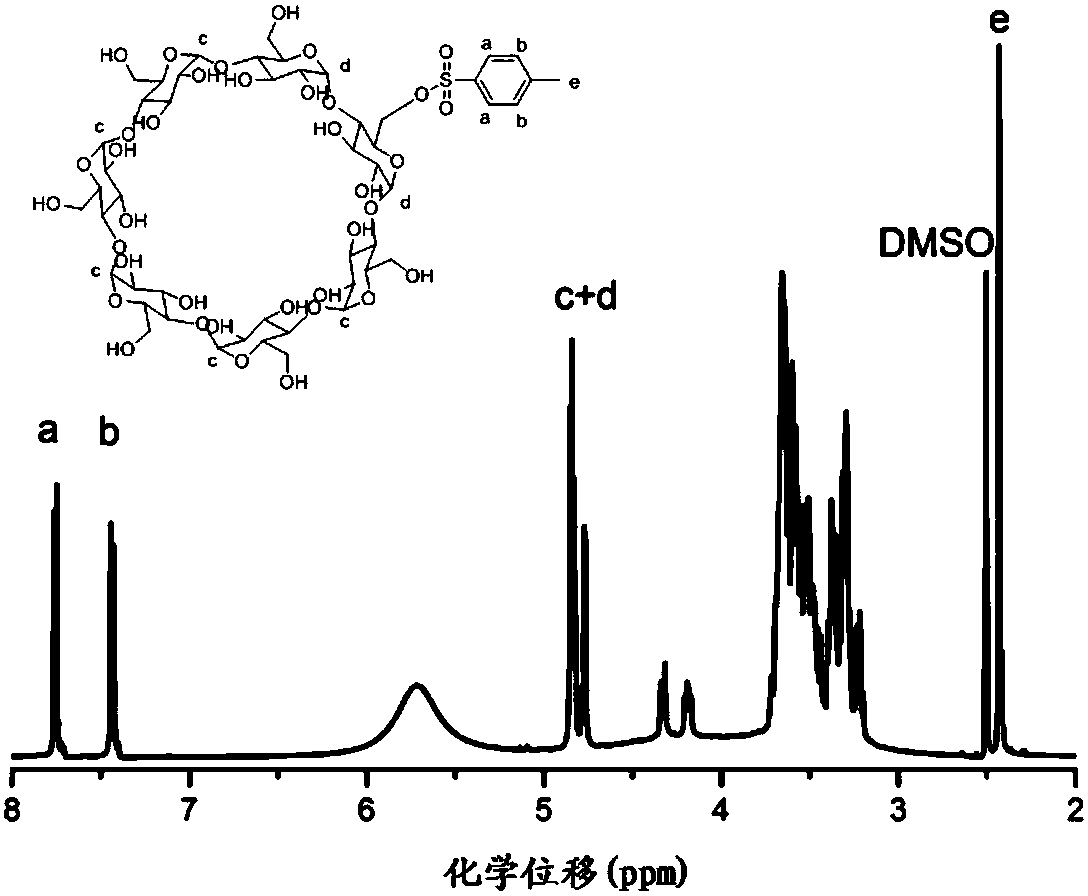
[0048] Suspend 60g of β-cyclodextrin in 500mL of water, add 7g of saturated sodium hydroxide solution dropwise at 0°C until the solution is clear and transparent; keep 0°C, dissolve 15g of p-toluenesulfonyl chloride in 10mL of acetonitrile, dropwise Add the above solution, react at room temperature for 2 hours; place at 4°C overnight, filter the solid with suction and recrystallize with water 3 times to obtain β-cyclodextrin activated by p-toluenesulfonyl chloride, see figure 1 .
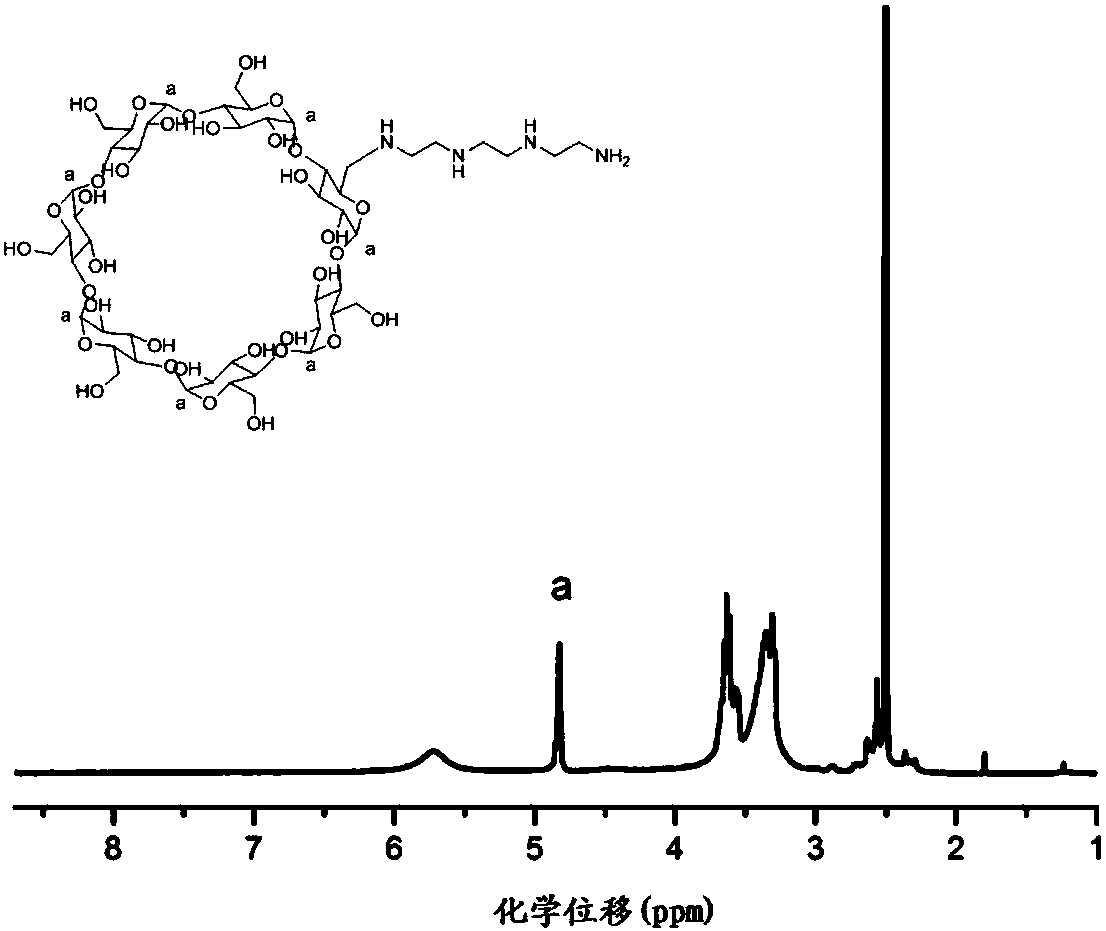
[0049] Dissolve 1.5g of β-cyclodextrin activated by p-toluenesulfonyl chloride in 20mL of DMF, heat to 80°C, add 3.5mL of triethylenetetramine and 2mL of triethylamine in sequence; after 48 hours of reaction, precipitate with ether three times, white The solid was dried in a vacuum oven to obtain an aminated β-cyclodextrin, see figure 2 .
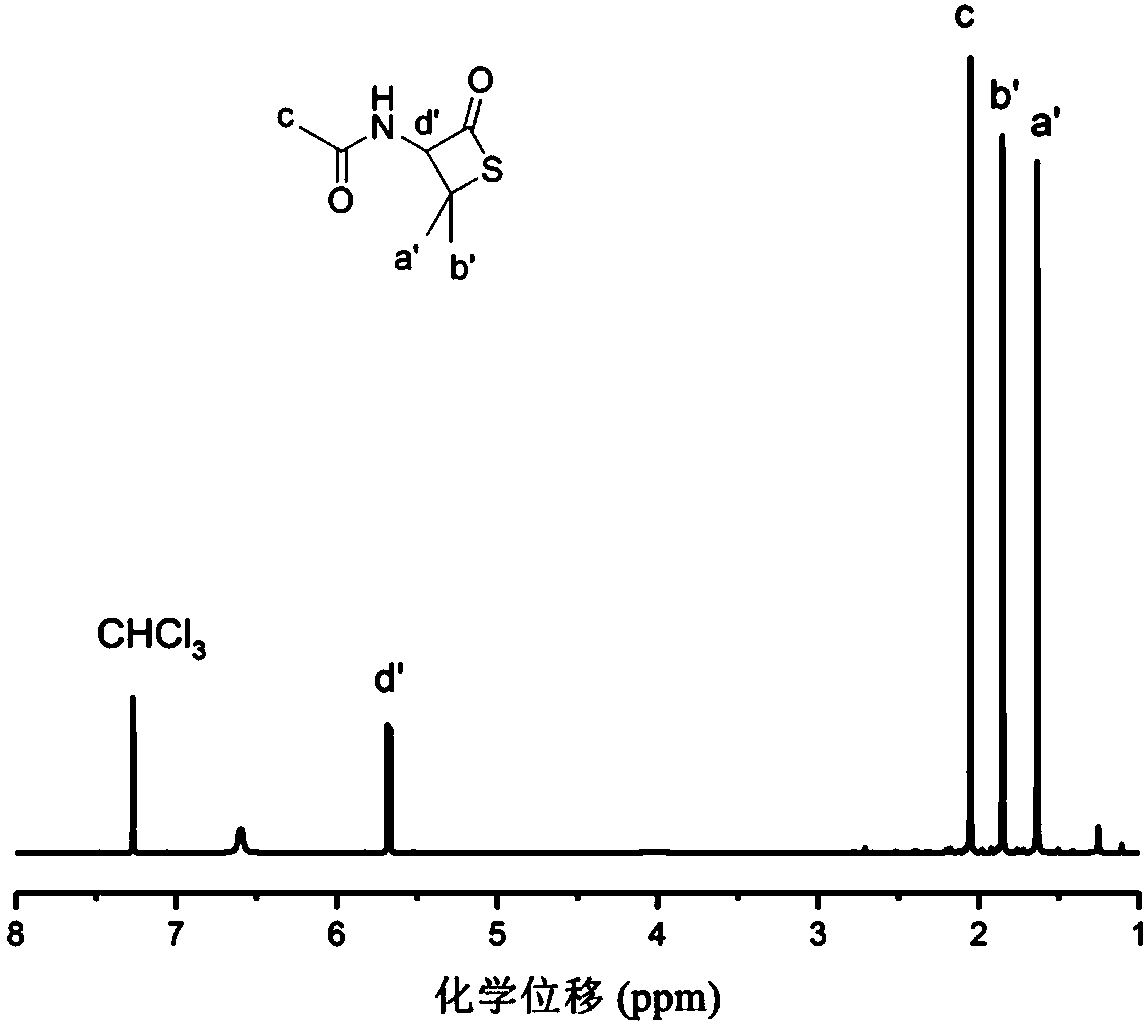
[0050] Dissolve 3.5g of D-penicillamine in 20mL of dimethyl sulfoxide, cool to 0°C, add 15mL of acetic anhydride in dimethyl sulfoxide dropwise, keep at -20°C...
Embodiment 2
[0054] Suspend 60g of α-cyclodextrin in 500mL of water, add 7g of saturated sodium hydroxide solution dropwise at 0°C until the solution is clear and transparent; keep 0°C, dissolve 15g of p-toluenesulfonyl chloride in 10mL of acetonitrile, Add the above solution, react at room temperature for 2 hours; place at 4°C overnight, filter the solid with suction and recrystallize 3 times with water to obtain α-cyclodextrin activated by p-toluenesulfonyl chloride.
[0055] Dissolve 3 g of α-cyclodextrin activated by p-toluenesulfonyl chloride in 25 mL of DMF, heat to 80 ° C, add 3 mL of ethylenediamine and 2 mL of 4-dimethylaminopyridine in sequence, react for 24 hours, and precipitate three times with acetonitrile, white solid Dry in a vacuum oven to obtain aminated α-cyclodextrin.
[0056] Dissolve 5g of D-penicillamine in 20mL of DMF, add 15mL of acetic anhydride in DMF dropwise, and keep it at 80°C for 2 hours. After the reaction is complete, remove the solvent with a rotary evapo...
Embodiment 3
[0060] Suspend 60g of α-cyclodextrin in 500mL of water, add 7g of saturated sodium hydroxide solution dropwise at 0°C until the solution is clear and transparent; keep 0°C, dissolve 15g of p-toluenesulfonyl chloride in 10mL of acetonitrile, Add the above solution, react at room temperature for 2 hours; place at 4°C overnight, filter the solid with suction and recrystallize 3 times with water to obtain α-cyclodextrin activated by p-toluenesulfonyl chloride.
[0061] Dissolve 3 g of cyclodextrin activated by p-toluenesulfonyl chloride in 25 mL of DMF, heat to 80 °C, and add 3 mL of NH 2 -CH 2 CH=CHCH 2 -NH 2 and 2 mL of triethylamine, reacted for 36 hours, precipitated with methanol three times, and dried the white solid in a vacuum oven to obtain aminated α-cyclodextrin.
[0062] Dissolve 15g of D-penicillamine in 20mL of toluene, add 150mL of acetic anhydride in toluene drop by drop, keep the reaction at 60°C for 20 hours, after the reaction is complete, remove the solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com