A kind of preparation method and application of dialdehyde base cellulose salenmx catalyst
A cellulose and dialdehyde-based technology, which is applied in the field of preparation of dialdehyde-based cellulose SalenMX catalysts, can solve the problems of easy shedding of catalysts, non-renewable monomers, harsh preparation conditions, etc., and achieves good environment, easy operation, and reuse. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: dialdehyde base cellulose SalenMn (OAc) 2 The preparation method of catalyst, concrete operation is as follows:
[0030] (1) Add 50mL H to a 250mL three-necked flask 2 O and 5.0013g of NaIO with a purity of 99.5% 4 , after stirring for 30min, then dropwise add concentrated H with a mass fraction of 98% to the three-neck flask 2 SO 4 , adjust the pH to 2, then add 5.0008g microcrystalline cellulose to the three-necked flask, and stir and react at 30°C for 2h;
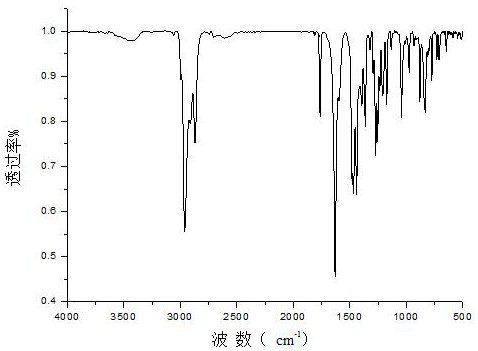
[0031] (2) Pour 20mL of ethylene glycol into the solution after the reaction in step (1) and stir for 20 minutes to form a white precipitate. After filtering, wash the precipitate with water for 5 times, and vacuum dry the washed precipitate at 23°C for 12 h , 4.9412g of dialdehyde-based cellulose was obtained, and the product was characterized by Fourier transform infrared spectroscopy (FTIR). figure 1 , it can be seen that -COH exists on the chain of cellulose;
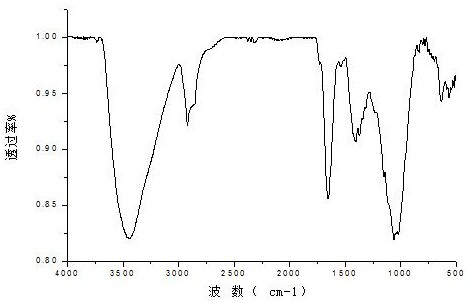
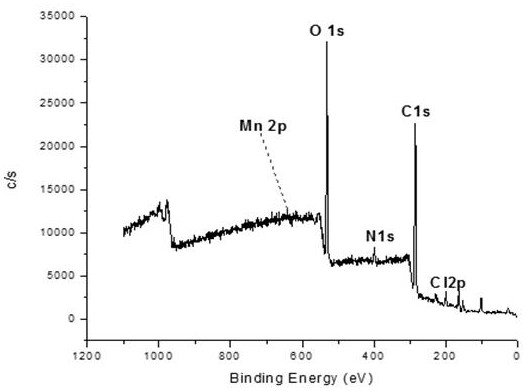
[0032](3) Add 4.9412g of dial...
Embodiment 2
[0035] Embodiment 2: the preparation method of dialdehyde base cellulose SalenCoBr catalyst, specific operation is as follows:
[0036] (1) Add 50mL H to a 250mL three-necked flask 2 O and 10.3281 g of NaIO with a purity of 99.5% 4 , after stirring for 50 min, then dropwise add concentrated H with a mass fraction of 98% to the three-neck flask 2 SO 4 , adjust the pH to 3, then add 5.1608g nanocrystalline cellulose to the three-necked flask, and stir and react at 40°C for 3h;
[0037] (2) Pour 40mL of ethylene glycol into the solution after the reaction in step (1) and stir for 40 minutes to form a white precipitate. After filtering, wash the precipitate with water for 4 times, and dry the washed precipitate under vacuum at 23°C for 24 h , to obtain 5.0638g of dialdehyde-based cellulose;
[0038] (3) Add 5.0638g of dialdehyde-based cellulose and 100mL of ethanol obtained in step (2) into a three-necked flask at the same time and raise the temperature to 60°C. Add 15.1201g o...
Embodiment 3
[0041] Embodiment 3: Dialdehyde base cellulose SalenCrCl 2 The preparation method of catalyst, concrete operation is as follows:
[0042] (1) Add 100mL H to a 250mL three-neck flask 2 O and 10.3281 g of NaIO with a purity of 99.5% 4 , after stirring for 60min, then dropwise add concentrated H with a mass fraction of 98% to the three-neck flask 2 SO 4 Adjust the pH to 1, then add 10.2619 g of a mixture of microcrystalline cellulose and nano-microcrystalline cellulose to the three-necked flask, and stir and react at 20° C. for 4 h;
[0043] (2) Pour 30mL of ethylene glycol into the solution after the reaction in step (1) and stir for 60 minutes to form a white precipitate. After filtering, wash the precipitate with water for 3 times, and dry the washed precipitate under vacuum at 24°C for 8 h , to obtain 9.8214g of dialdehyde-based cellulose;
[0044] (3) Add 9.8214g of dialdehyde-based cellulose and 100mL of ethanol obtained in step (2) into a three-necked flask at the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com