Recombinant GLP-1 analogue Fc fusion protein optimized gene and application thereof
A GLP-1, fusion protein technology, applied in the direction of animal/human protein, fusion polypeptide, recombinant DNA technology, etc., can solve problems such as long-term maintenance of efficacy, lack of stability of cell lines, instability of foreign genes, etc. , to enhance the affinity, prolong the half-life in the body, and enhance the efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
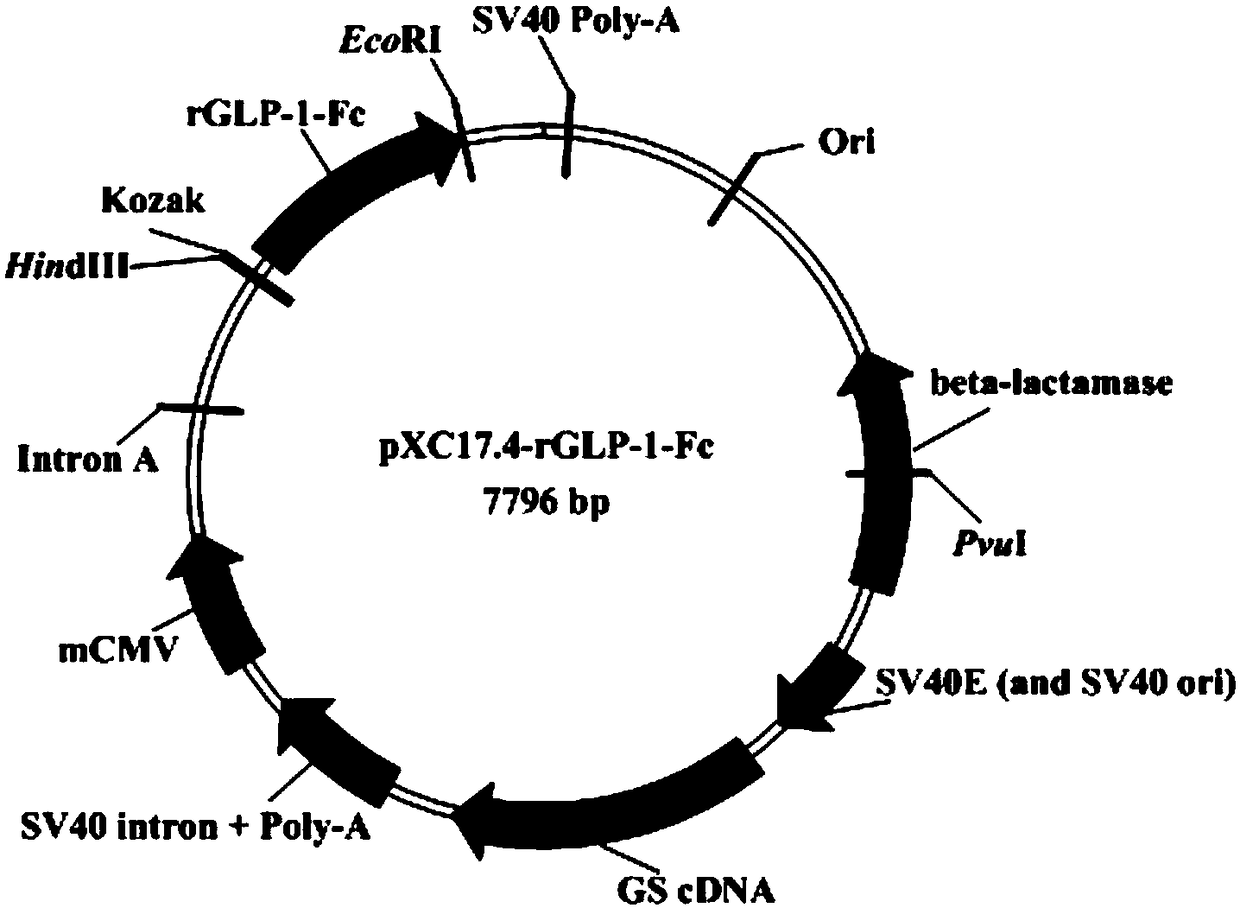
[0028] Example 1 Construction of recombinant expression plasmid pXC17.4-rGLP-1-Fc
[0029] 1. Full gene synthesis of recombinant GLP-1 analogue Fc fusion protein gene (rGLP-1-Fc)
[0030] According to the literature (Haryadi R, Ho S, Kok YJ, et al. Optimization of Heavy Chain and Light Chain Signal Peptides for High Level Expression of Therapeutic Antibodies in CHO Cells[J]. Plos One, 2015, 10(2): e0116878.) Through screening and optimization, the signal peptide A (SEQ ID NO:1, 19aa) was obtained, and according to the patent (A·M·Vick, R·L·Xiaomilican, W·Gleissner.GLP -1 analogue fusion protein, CN 1802386B[P].2010.) published the amino acid sequence of GLP-1 analogue (total 275aa), combining the two amino acid sequences, according to the Chinese hamster ovary cell (CHO cell) Optimize the strategy, optimize the sequence, add HindIII and Kozak sequences to the 5'end of the sequence, add double stop codons and EcoRI to the 3'end of the sequence, and obtain the GLP-1 analogue Fc fusi...
Embodiment 2
[0033] Example 2 Stable Cell Pool Screening
[0034] 1. pXC17.4-rGLP-1-Fc electroporation
[0035] Use the PvuI restriction site to linearize the plasmid pXC17.4-rGLP-1-Fc, and after purification, filter sterilization and sterility testing, the linearized plasmid pXC17.4-rGLP-1-Fc (concentration 0.4 ug / uL). Also prepare 2×10 7 The exponential growth phase CHOK1SV GS-KO cells are added to two shock cups, each electrode cup contains 1×10 7 cells, the volume is 0.7mL, each add 100μL of 0.4 ug / uL linearized DNA into the electroporation cup, and linearize plasmid pXC17 according to the preset program (pulse 300V, 900μF, resistance ∞, exponential wave, 4mm gap). 4-rGLP-1-Fc was electrotransfected into CHOK1SV GS-KO recipient cells.
[0036] 2. The transfected cells are spread on 96-well plates and pool screening
[0037] Resuspend the cells after the electric shock in a 1L conical flask containing 200mL CD CHO AGT medium, mix gently, pour into the sample tank, use a drain gun to draw the ...
Embodiment 3
[0040] Example 3 Monoclonal ClonePix2 screening
[0041] 1.Pool semi-solid medium
[0042] 1)Pool semi-solid medium culture formula
[0043] Table 1 The formula of semi-solid medium for cell Pool cloning
[0044]
[0045] Blow and suck evenly at room temperature and wait for the bubbles to disappear slowly.
[0046] 2)Pool semi-solid medium
[0047] According to the 150cells / 2mL semi-solid medium per well after plating, add the required amount of cell liquid to the semi-solid medium. Use a pipette to gently mix the semi-solid medium with the cells, and let it stand at room temperature for a few minutes to make the large bubbles disappear or break. Use a pipette to suck up 2mL of the above mixed cell liquid and slowly add it to each hole in the six-well plate. Be careful not to mix in air bubbles. If there are air bubbles, just pick them up gently. After aliquoting and spreading, cover the lid and place at 37℃, 5% CO 2 Stand still and cultivate.
[0048] 2. ClonePix2 selects a single cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com