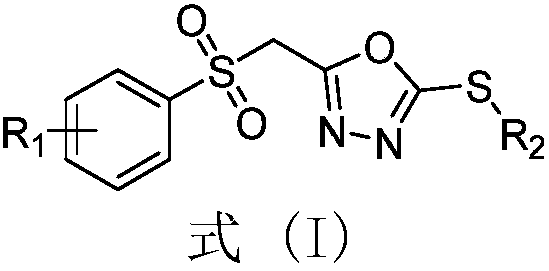
2,5-substituent-1,3,4-oxadiazole sulfone derivative as well as preparation method and application thereof
A technology of oxadiazole sulfones and oxadiazoles, which is applied to 2,5-substituent-1,3,4-oxadiazole sulfone derivatives, their preparation and application fields, and can solve the problems of microorganisms, insects and pests Natural enemies poisoning, endangering a variety of biological resources, insufficient control effect, etc., to achieve the effects of excellent activity, simple structure and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
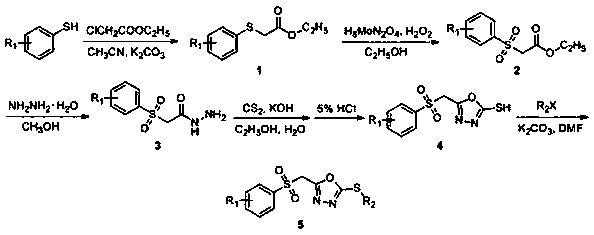
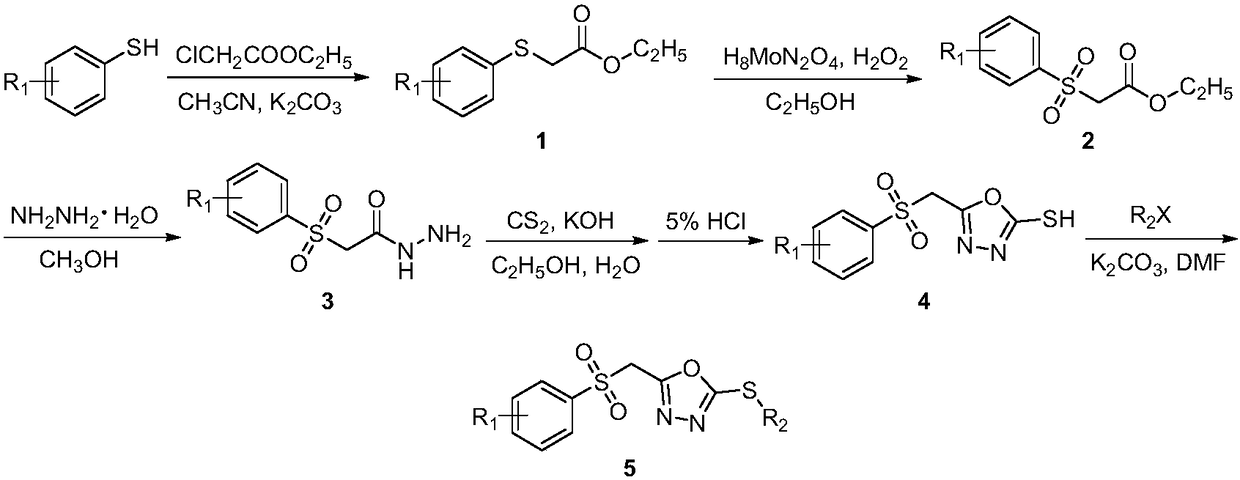
[0032] A preparation method of 2-methylthio-5-(((4-fluorophenyl)sulfone)methyl)-1,3,4-oxadiazole (compound 5a), comprising the following steps:
[0033] (1) Synthesis of ((4-fluorophenyl) thio) ethyl acetate
[0034] Add 4-fluorothiophenol (0.21mol) and potassium carbonate (0.23mol) into a 250mL three-neck flask, add 100mL of acetonitrile, then add ethyl chloroacetate (0.22mol) dropwise into the three-necked flask, and turn on the heating , TLC tracking reaction (developing agent: sherwood oil: ethyl acetate=10:1), after the reaction finishes, suction filtration, the acetonitrile in the filtrate is removed under reduced pressure to obtain intermediate ((4-fluorophenyl) thio) acetic acid ethyl ester.
[0035] (2) Synthesis of ((4-fluorophenyl) sulfone group) ethyl acetate
[0036]The synthetic intermediate ((4-fluorophenyl)thio)ethyl acetate (43mmol) was added to a 100mL three-necked flask, 20mL of absolute ethanol was added, and ammonium molybdate (2.2mmol) was dissolved in ...
Embodiment 2
[0044] A preparation method of 2-ethylthio-5-(((4-fluorophenyl)sulfone)methyl)-1,3,4-oxadiazole (compound 5b), comprising the following steps:
[0045] (1) Synthesis of ((4-fluorophenyl) thio) ethyl acetate
[0046] Add 4-fluorothiophenol (0.21mol) and potassium carbonate (0.23mol) into a 250mL three-neck flask, add 100mL of acetonitrile, then add ethyl chloroacetate (0.22mol) dropwise into the three-necked flask, and turn on the heating , TLC tracking reaction (developing agent: sherwood oil: ethyl acetate=10:1), after the reaction finishes, suction filtration, the acetonitrile in the filtrate is removed under reduced pressure to obtain intermediate ((4-fluorophenyl) thio) acetic acid ethyl ester.
[0047] (2) Synthesis of ((4-fluorophenyl) sulfone group) ethyl acetate
[0048] The synthetic intermediate ((4-fluorophenyl)thio)ethyl acetate (43mmol) was added to a 100mL three-necked flask, 20mL of absolute ethanol was added, and ammonium molybdate (2.2mmol) was dissolved in ...
Embodiment 3
[0056] A preparation method of 2-benzylthio-5-(((4-fluorophenyl)sulfone)methyl)-1,3,4-oxadiazole (compound 5c), comprising the following steps:
[0057] (1) Synthesis of ((4-fluorophenyl) thio) ethyl acetate
[0058] Add 4-fluorothiophenol (0.21mol) and potassium carbonate (0.23mol) into a 250mL three-neck flask, add 100mL of acetonitrile, then add ethyl chloroacetate (0.22mol) dropwise into the three-necked flask, and turn on the heating , TLC tracking reaction (developing agent: sherwood oil: ethyl acetate=10:1), after the reaction finishes, suction filtration, the acetonitrile in the filtrate is removed under reduced pressure to obtain intermediate ((4-fluorophenyl) thio) acetic acid ethyl ester.
[0059] (2) Synthesis of ((4-fluorophenyl) sulfone group) ethyl acetate
[0060] The synthetic intermediate ((4-fluorophenyl)thio)ethyl acetate (43mmol) was added to a 100mL three-necked flask, 20mL of absolute ethanol was added, and ammonium molybdate (2.2mmol) was dissolved in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com