Glucose oxidase mutant with improved heat resistance
A technology of glucose oxidase and mutants, which is applied in the direction of oxidoreductase, enzyme, and microbial-based methods, can solve the problems of poor thermal stability, application limitations, and increased equipment investment of glucose oxidase, so as to be beneficial to wide application, Improved heat resistance and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
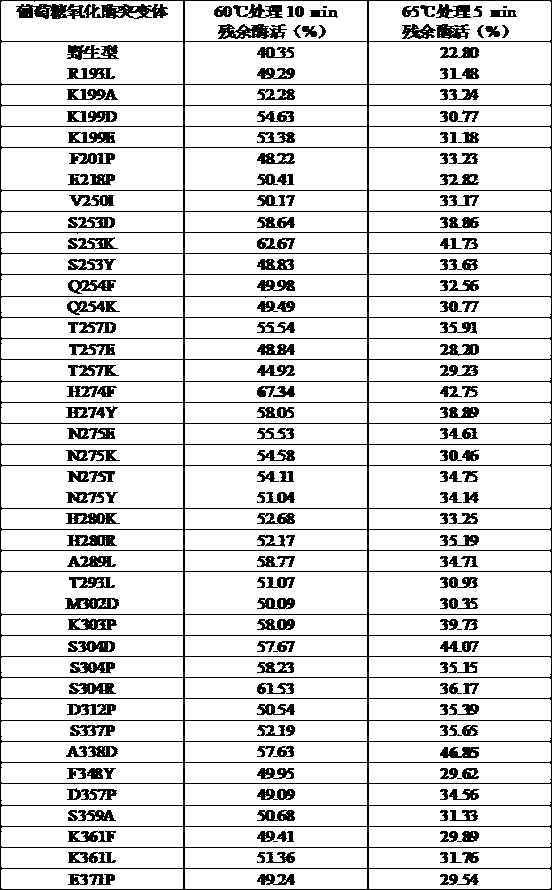
Image
Examples
Embodiment 1
[0056] Example 1 Obtainment of glucose oxidase heat-resistant single point mutant
[0057] 1.1 Amplification of the glucose oxidase gene
[0058] Aspergillus niger ( Aspergillus niger ) genome as a template for PCR amplification, PCR primers GOD-F1 and GOD-R1 are as follows:
[0059] GOD-F1: GGTATTGAGGCATCTTTGTTGAC
[0060] GOD-R1: TTATTGCATAGAAGCGTAATC
[0061] The PCR product was recovered by gel, connected to the pEASY-T vector, transformed into Escherichia coli DH5α, and the correct transformant was picked for sequencing. Sequencing results showed that the nucleotide sequence of the amplified gene fragment was SEQ ID NO: 2, and the encoded amino acid sequence was SEQ ID NO: 1. Through NCBI BLAST comparison, it was found that the sequence similarity between SEQ ID NO: 1 and the glucose oxidase from Aspergillus niger was as high as 100%, so it was confirmed that the gene obtained by PCR was the glucose oxidase gene and named GOD.
[0062] Amplification and Synthesis of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com