Burning rate catalyst and preparation method thereof
A burning rate catalyst and oxide technology, which is applied in the direction of offensive equipment, non-explosive/non-thermal agent components, explosives, etc., can solve the problems that the burning rate catalyst cannot be completely solved, and the catalytic efficiency has not been maximized, etc., to achieve excellent Catalytic activity, good thermal and electrical conductivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Follow the steps below to prepare loaded Co 3 o 4 The graphene airgel catalyst:
[0032] 1. Add 30mg of Co with a diameter of 30nm 3 o 4 Nanoparticles were added to 10 mL of graphene aqueous solution with a concentration of 3 mg / mL and ultrasonically dispersed for 15 min. Then add 1 mL of ammonia water with a mass fraction of 25%, ultrasonically disperse for 10 min; add the obtained solution into a glass bottle with a cap, tighten the cap, put it in an oil bath, raise the temperature to 90°C, and let stand for 24 h; The load has Co 3 o 4 Cylindrical graphene hydrogels of nanoparticles.
[0033] 2. Rinse the above hydrogel several times with deionized water, then put the obtained hydrogel into 20 mL of 10% volume concentration ethanol aqueous solution, let it stand for 12 hours, and exchange the liquid in the hydrogel.
[0034] 3. Take out the above-mentioned hydrogel and put it into the freeze-drying device for 24 hours to obtain a cylindrical gel loaded with Co ...
Embodiment 2
[0037] The graphene airgel catalyst loaded with NiO was prepared according to the following steps:
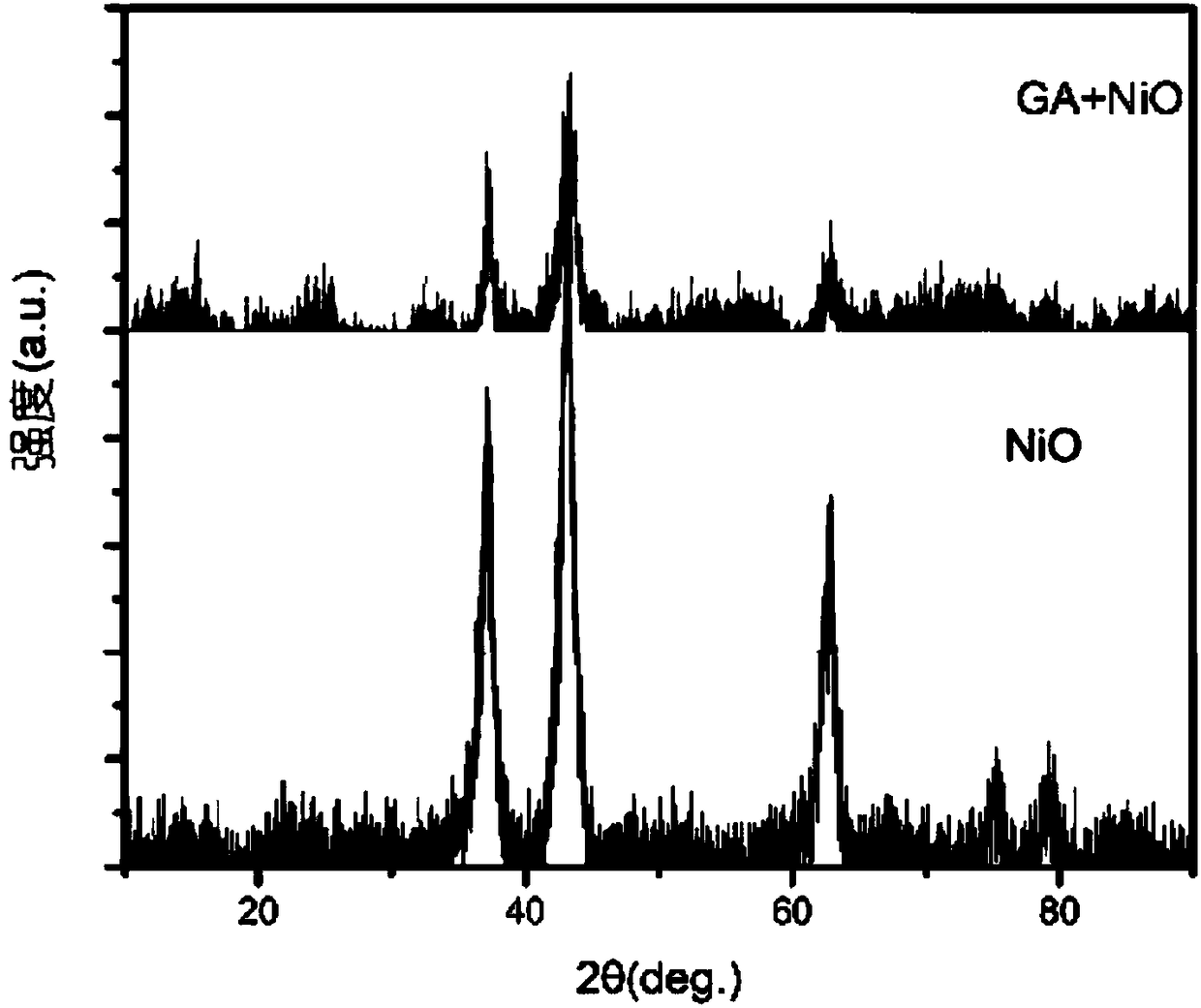
[0038] 1. Add 50 mg of NiO nanoparticles with a diameter of 30 nm into 10 mL of graphene aqueous solution with a concentration of 5 mg / mL, and ultrasonically disperse for 15 min; then add 1 mL of ethylenediamine aqueous solution with a concentration of 0.1 mol / mL, and ultrasonically disperse for 10 min; The obtained solution was added into a glass bottle with a cap, the cap was tightened, put into an oil bath, heated to 80° C., and allowed to stand for 12 hours; a cylindrical graphene hydrogel loaded with NiO nanoparticles was obtained.
[0039] 2. Rinse the above hydrogel several times with deionized water, then put the obtained hydrogel into 20 mL of 10% volume concentration ethanol aqueous solution, let it stand for 12 hours, and exchange the liquid in the hydrogel.
[0040] 3. Take out the hydrogel and put it into a freeze-drying device for 24 hours to obtain a cylindrical ...
Embodiment 3
[0044] The CuO-supported graphene airgel catalyst was prepared according to the following steps:
[0045] 1. Add 50mg of CuO nanoparticles with a diameter of 40nm to 10mL of graphene aqueous solution with a concentration of 5mg / mL, ultrasonically disperse for 15min; then add 1mL of NaHSO with a concentration of 0.1mol / mL 3 Aqueous solution, ultrasonic dispersion for 10min; put the obtained solution into a glass bottle with a cap, tighten the cap, put it in an oil bath, raise the temperature to 90°C, and let it stand for 24h; obtain cylindrical graphite loaded with CuO nanoparticles Alkene hydrogel.
[0046] 2. Rinse the above hydrogel several times with deionized water, then put the obtained hydrogel into 20 mL of 10% volume concentration ethanol aqueous solution, let it stand for 12 hours, and exchange the liquid in the hydrogel.
[0047] 3. Take out the above-mentioned hydrogel and put it into a freeze-drying device for 24 hours to obtain a cylindrical graphene airgel loaded ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com