Lipid droplet fluorescent probe and synthetic method and application thereof
A technology of fluorescent probes and lipid droplets, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of high cost, fewer fluorescent probes, cumbersome synthesis steps, etc., and achieve easy application and simple synthesis route , to achieve the effect of intracellular imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
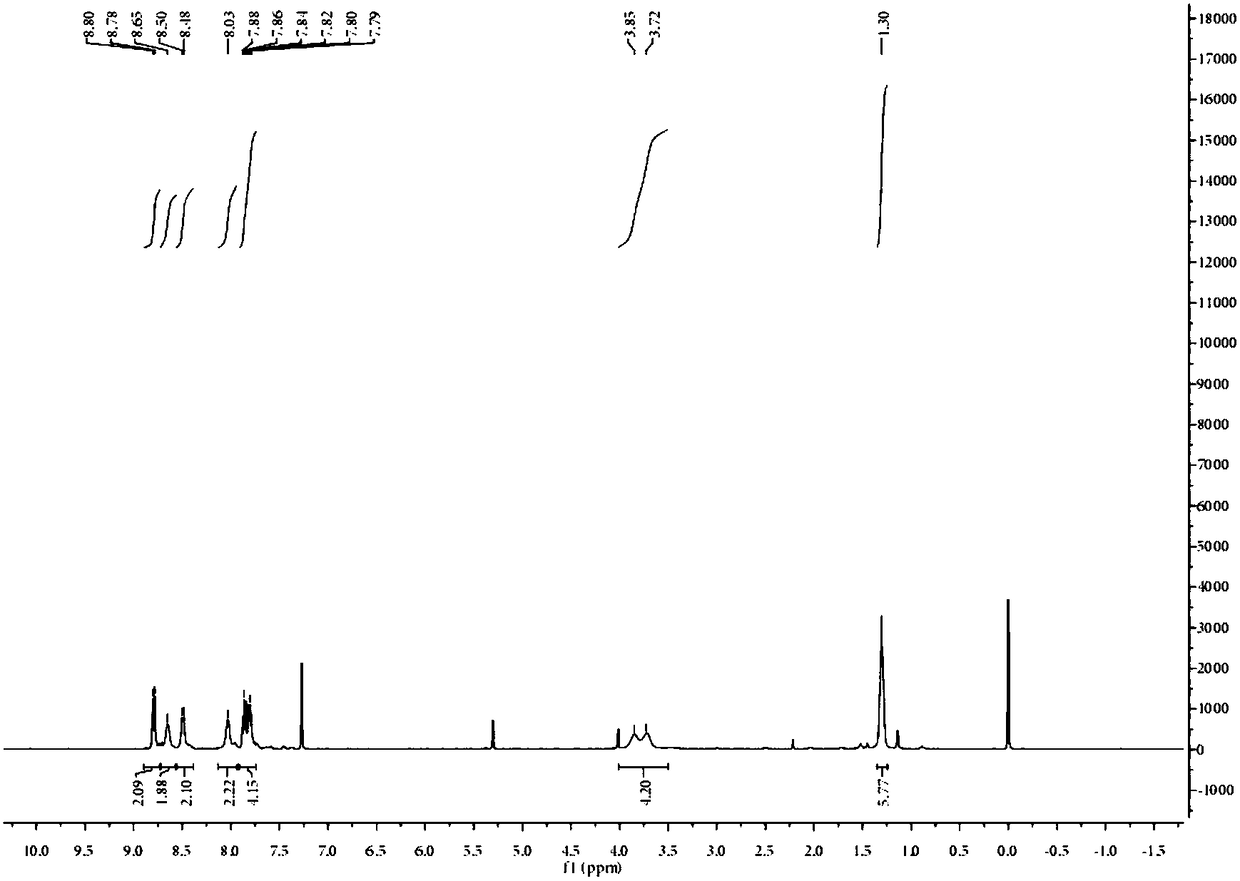
[0029] Example 1 Synthesis of PIA.
[0030] Dissolve 0.25g (1.2mmol) 9,10-phenanthrenequinone and 0.18g (1mmol) N,N-diethyl-4-aminobenzaldehyde in 20mL acetic acid, add 0.38g (5mmol) ammonium acetate as catalyst, The reaction was refluxed at 100 °C for 12 h under the protection of nitrogen. After the reaction, cool to room temperature, pour into ice water and stir, adjust the pH to neutral, filter with suction, and dry in a vacuum oven to obtain the probe compound with a yield of 80%. The proton magnetic spectrum of the probe compound is as follows figure 1 shown.
Embodiment 2
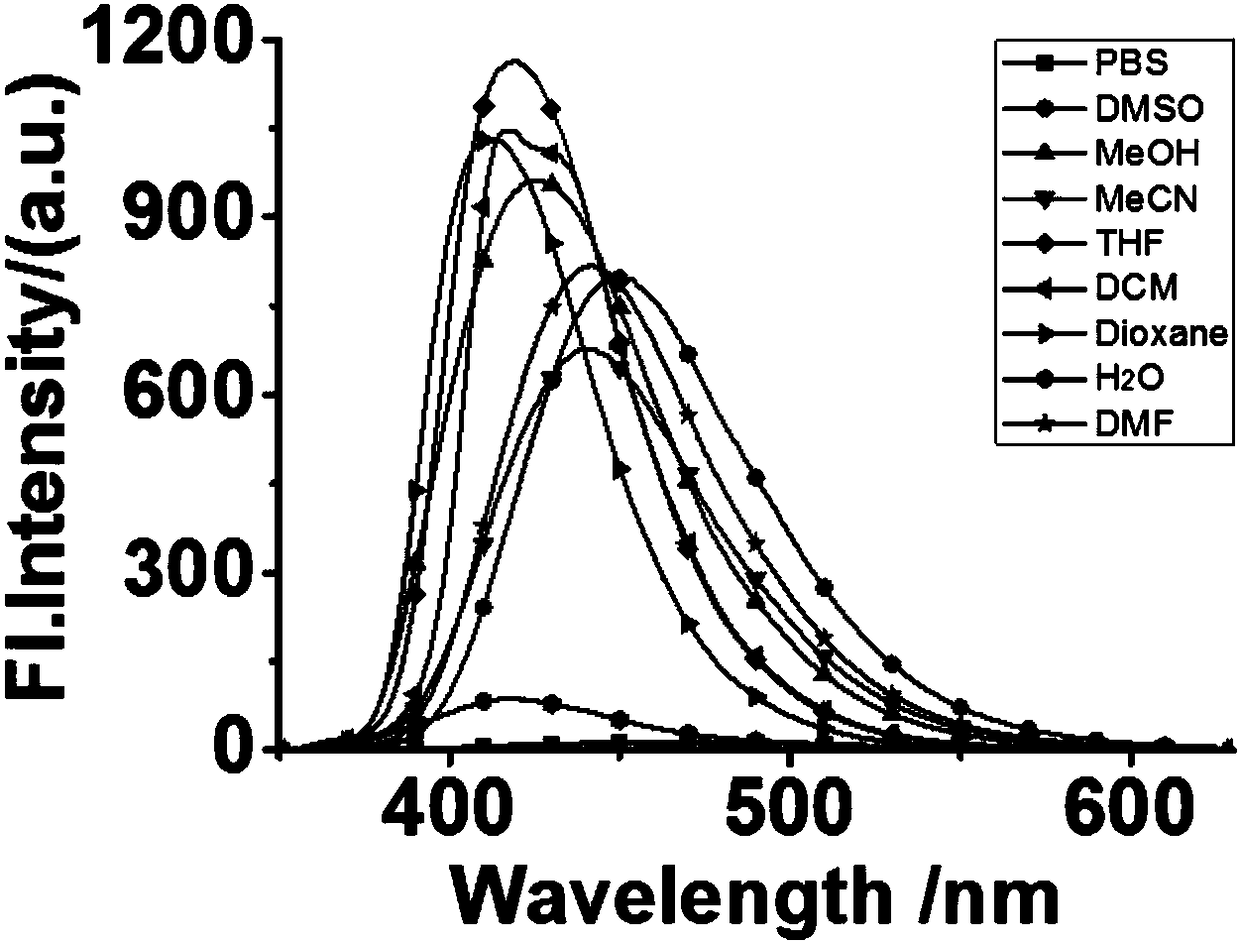
[0031] Example 2 Solvation effect of PIA.
[0032] The probe in Example 1 is dissolved in dimethyl sulfoxide, and it is prepared that the concentration is 10 -3 Mother liquor of M. Take 50 μL of the above mother solution and add it to nine identical 5mL volumetric flasks, and use DMF, DMSO, THF, MeCN, PBS, MeOH, DCM, H 2 O, Dioxane was diluted to 5mL, and then carried out fluorescence detection, the results are shown in figure 2 :Depend on figure 2 It can be seen that the fluorescence intensity of the probe in the organic solution is much greater than that in the aqueous phase. This shows that when the detection environment is the water phase, the fluorescence intensity of the probe in the water is obviously weaker, so that the probe can be better used in the detection of the human body environment, greatly reducing the harmfulness of the probe to the human body, and also eliminating self-disruptive.
Embodiment 3
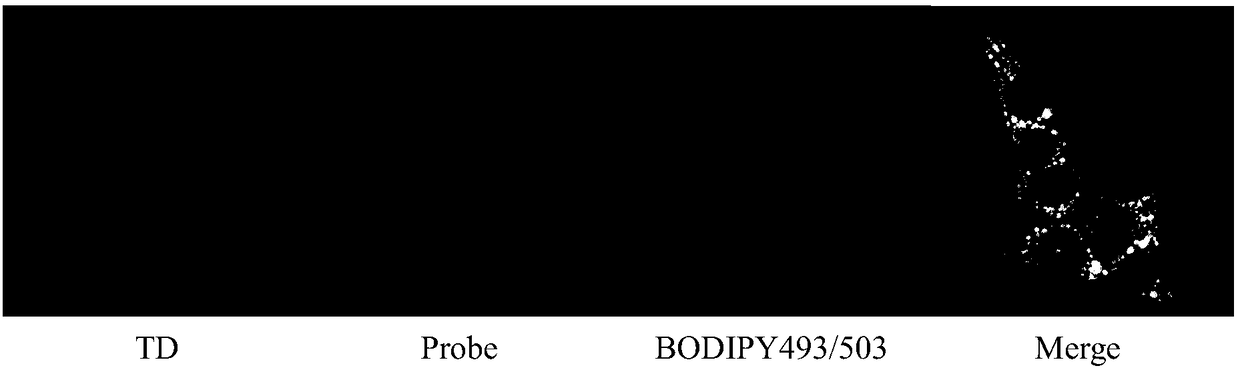
[0033] Example 3 Cellular colocalization of PIA.
[0034] The probe in Example 1 was made into a 1 mM stock solution with DMSO, and 5 μL of the stock solution was diluted with 1 mL of medium during staining to prepare a staining solution with a final concentration of 5 μM. The inoculated cells were incubated in the staining solution at 37 ºC for 30 min, washed 3 times with PBS, and the adherent cells were placed on a glass slide; then fluorescence imaging was performed with a fluorescence microscope, the excitation wavelength was 405nm, and the emission band was 425- 475nm. At the same time, co-localization experiments were carried out with a commercial lipid droplet probe (BODIPY493 / 503), with an excitation wavelength of 488 nm and an emission band of 500-550 nm. Images under a confocal fluorescence microscope as image 3 As shown, from left to right are bright field imaging, PIA probe imaging, BODIPY493 / 503 probe imaging, bright field, PIA probe and BODIPY493 / 503 probe sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com