Radix panacis quinquefolii controlreference extract as well as preparation method and application thereof
A technology of American ginseng extract and American ginseng, applied in the field of American ginseng reference extract and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: Preparation of American ginseng reference extract
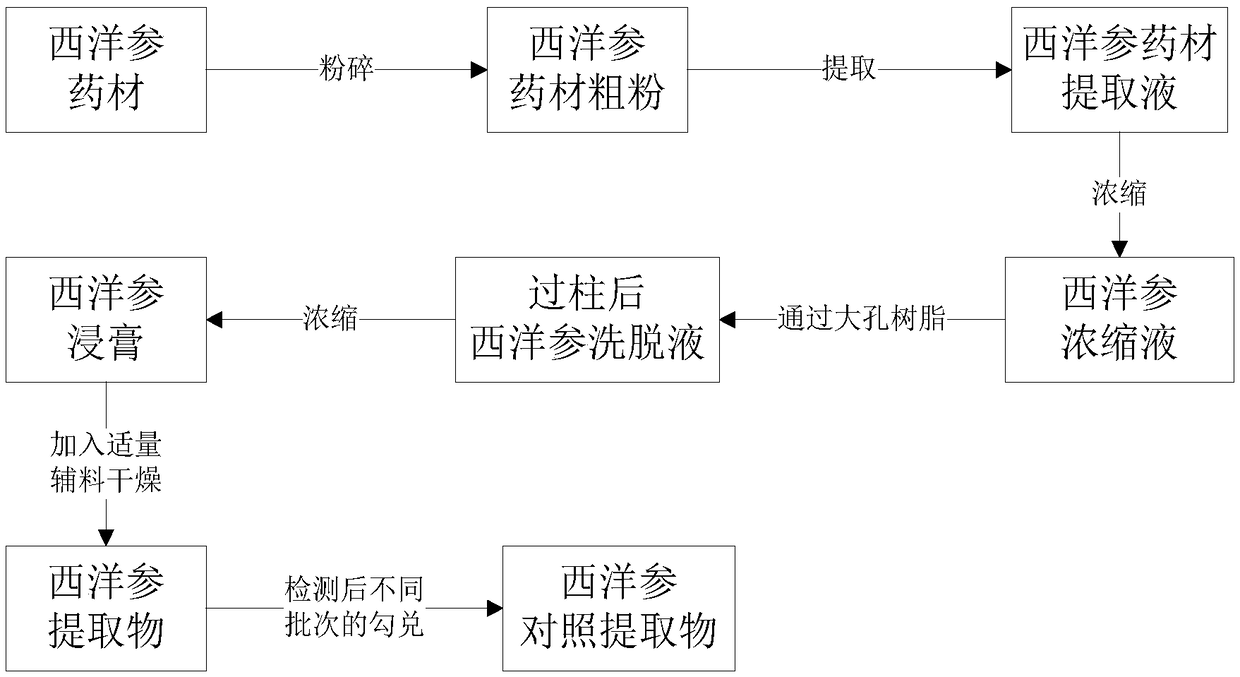
[0081] The present embodiment provides the preparation method of American ginseng reference extract of the present invention, and its method flowchart is as follows figure 1 shown.
[0082] One: extract
[0083] Select American ginseng medicinal materials (Canada, authentic medicinal materials) and make them into powders. The medicinal materials powders are passed through a No. 2 sieve (850±29 μm, 24 meshes), and then add ethanol with a volume 10 times its mass (that is, the solid-liquid ratio is 1:10 (w / V)), after flash extraction for 1 min, filter with medium-speed qualitative filter paper, collect filter residue 1 and filtrate 1, filter residue 1 is extracted twice in the same way, and combine the filtrate collected for the second extraction with filtrate 1 to obtain the extract , the extract is evaporated to dryness to obtain a dry paste;
[0084] Two: Preparation of American ginseng extract
[0...
Embodiment 2
[0096] The character analysis of embodiment 2 American ginseng control extract
[0097] 1. Apparent state: the American ginseng reference extract obtained in Example 1 is yellow gray powder.
[0098] 2. Moisture determination: according to the 2015 edition Chinese Pharmacopoeia Appendix IX G (reduced pressure drying method). The test result shows that the water content of the control extract of American ginseng is 3.91%.
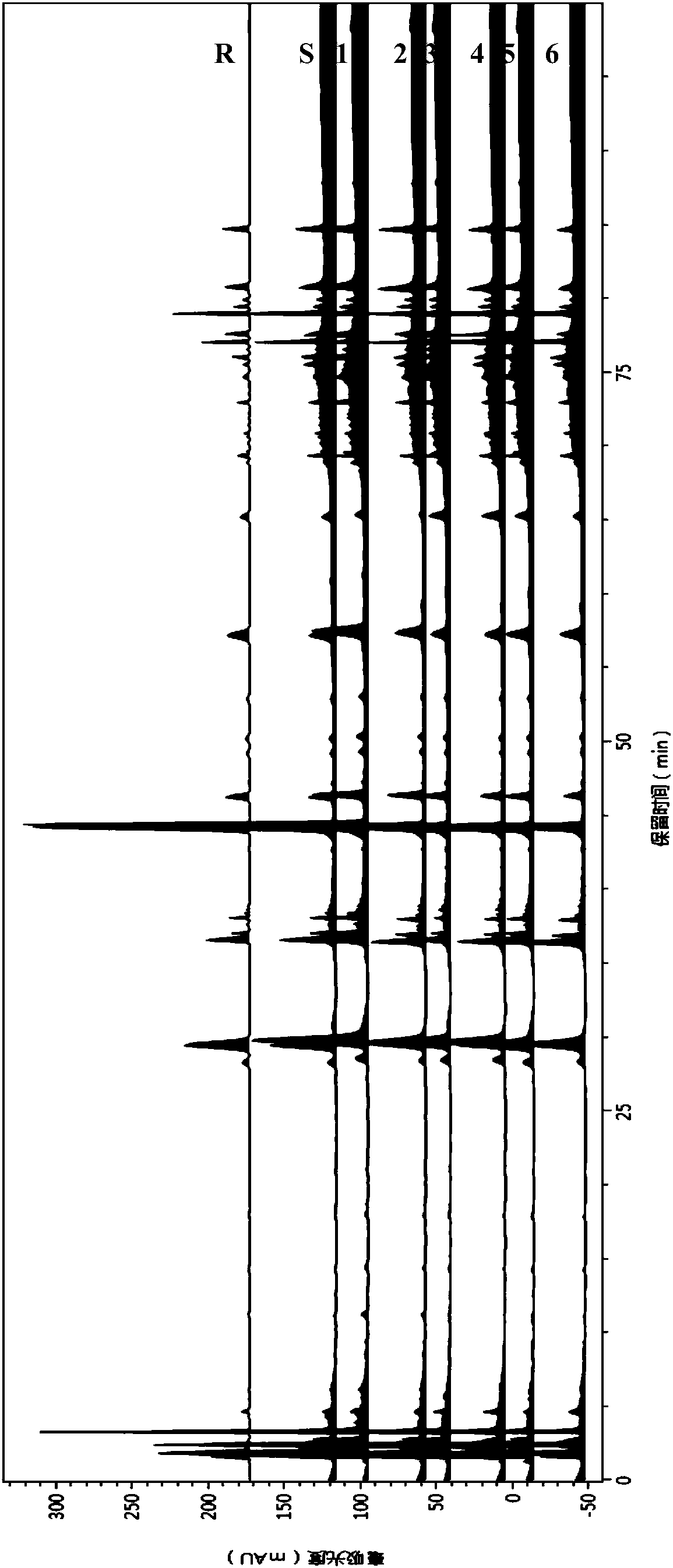
[0099] 3. Consistency test: 3 batches of American ginseng reference extracts were prepared according to the method in Example 1, and the thinness of ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd between each batch was measured. The layer chromatography detection result difference is very little, and the fluorescent spots of ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd all have obvious display, and distribution is very consistent; Through high performance liquid chromatography detec...
Embodiment 3
[0108] This example provides the application of the American ginseng control extract prepared in Example 1.
[0109] Commercial medicinal materials were analyzed by thin-layer chromatography and high-performance liquid chromatography with chemical reference substance and American ginseng reference extract as reference substances.
[0110] 1 TLC
[0111] 1.1 Sample preparation
[0112] Chemical reference solution: Precisely weigh ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc, and ginsenoside Rd, respectively, and prepare 0.2 mg / ml solutions with methanol.
[0113] American ginseng control extract solution: densely weigh the American ginseng extract reference substance, add methanol for ultrasonic extraction for 15 minutes, constant volume, prepare a solution with a concentration of 3 mg / ml, filter the membrane, and take the filtrate as the extract reference substance solution.
[0114] Medicinal material test solution: Accurately weigh 1g of American ginsen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com