Self-plasticizing fluorine-containing polymer lithium ion conductor, preparation method and application thereof
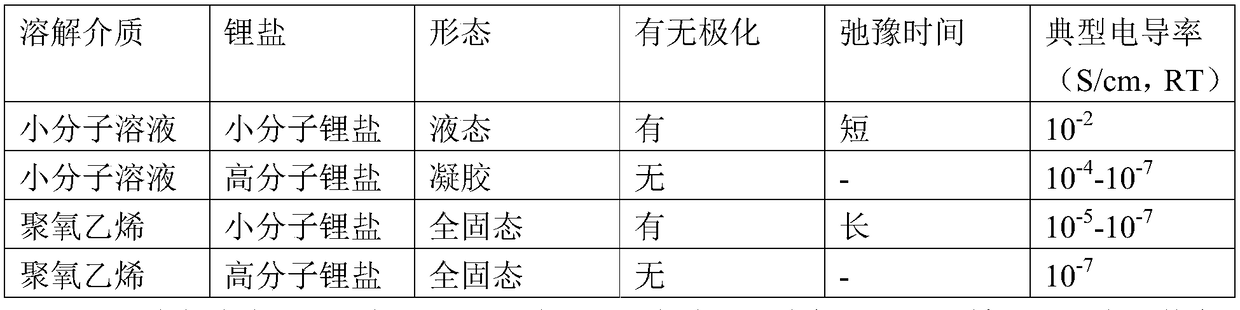
A polymer, self-plasticizing technology, applied in the field of polymer material technology and lithium batteries, can solve the problems of dispersion and interaction between polyoxyethylene materials and lithium salts that are rarely considered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
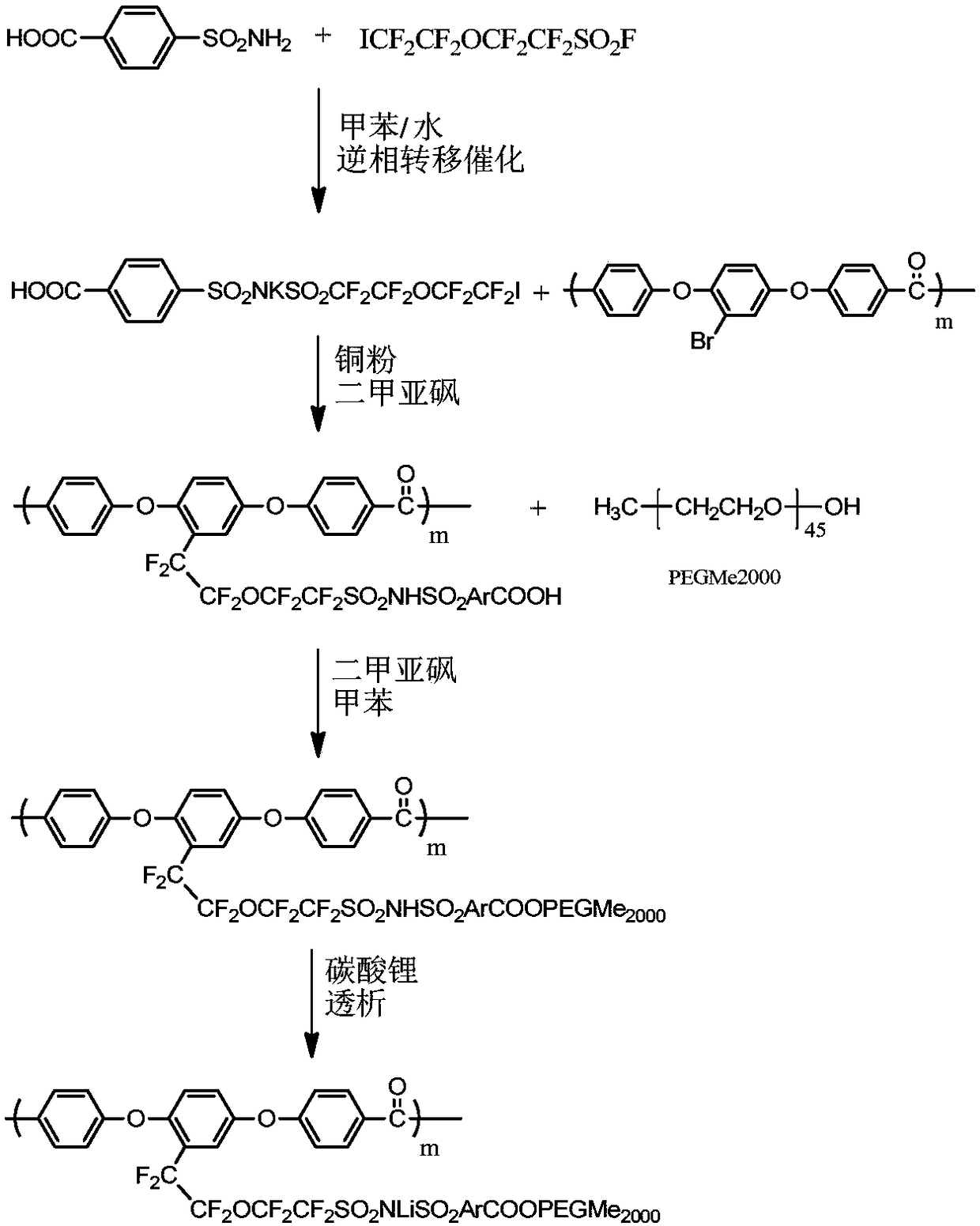
[0050] In this embodiment, the fluorine-containing polymer lithium ion conductor is a fluorine-containing polyetheretherketone resin (Li type) with side chain grafted PEGMe2000, and its synthetic reaction route is as follows figure 1 As shown, the details are as follows:
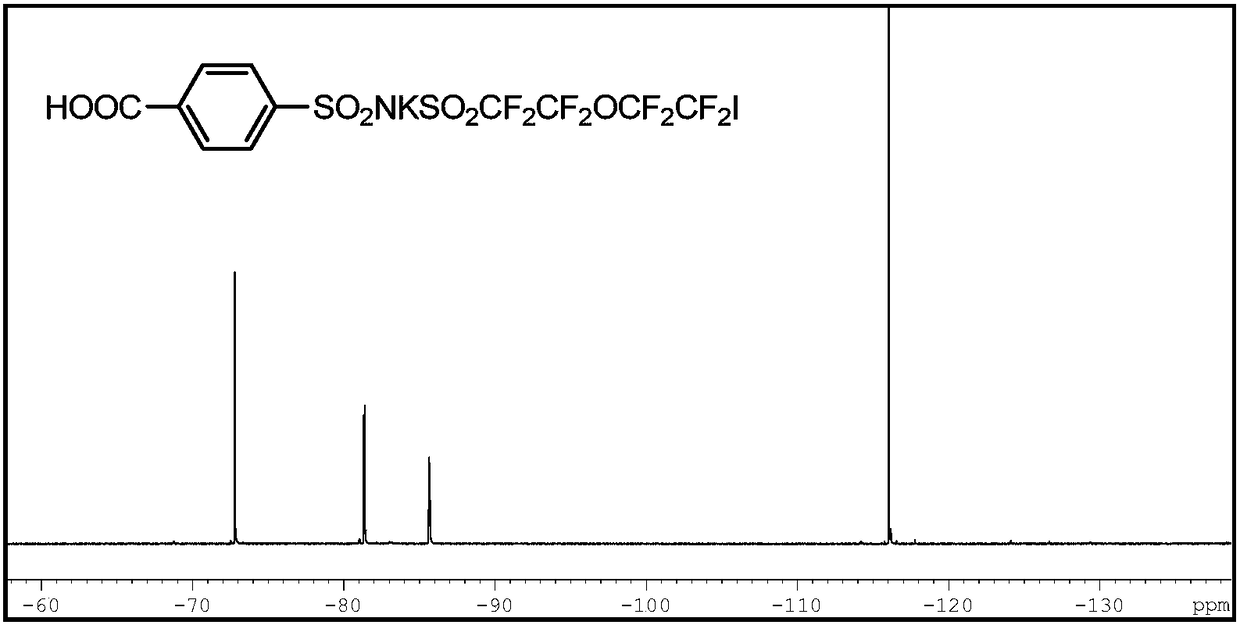
[0051] Step 1: Synthesis of potassium salt of p-carboxybenzenesulfo-(5-iodo-3-oxa-octafluoropentyl)sulfonylimide (ICF 2 CF 2 OCF 2 CF 2 SO 2 NKSO 2 ArCOOH)
[0052] Add 50ml of deionized water, 0.01mol of p-carboxybenzenesulfonamide, 0.02mol of potassium hydroxide, and 0.01mol of potassium carbonate into a 250ml three-necked flask, and mix well; when the temperature returns to room temperature, add 0.0005mol of octadecadecrown ether, stir and dissolve , 50 ml of toluene solution in which 0.01 mol of 5-iodo-3-oxa-octafluoropentylsulfonyl fluoride monomer was dissolved was added dropwise, and reacted for 0.5 hmin under stirring at 1000 rpm.
[0053] The reaction product was separated from the water phas...
Embodiment 2
[0063] Step 1: Synthesis of (4-potassium carboxylate-3-oxa-hexafluorobutyl)-sulfo-(5-iodo-3-oxa-octafluoropentyl)sulfonylimide potassium salt (ICF 2 CF 2 OCF 2 CF 2 SO 2 NKSO 2 CF 2 CF 2 OCF 2 COOK)
[0064] Add 50ml acetonitrile in 250ml there-necked flask, 0.01mol4-carboxy-3-oxa-hexafluorobutylsulfonamide (N 2 SO 2 CF 2 CF 2 OCF 2 COOH), 0.04mol of anhydrous potassium carbonate were mixed uniformly and heated to 60°C, and 0.01mol of 5-iodo-3-oxa-octafluoropentylsulfonyl fluoride monomer (ICF 2 CF 2 OCF 2 CF 2 SO 2 F) Reaction for 6h.
[0065] After the mixture was filtered, the mother liquor was rotary evaporated to remove acetonitrile, and the obtained yellow-white solid was recrystallized with deionized water to obtain the product ICF 2 CF 2 OCF 2 CF 2 SO 2 NKSO 2 CF 2 CF 2 OCF 2 COOK.
[0066] Step 2: Synthesis of fluorine-containing sulfonimide grafted polysulfone with carboxyl groups
[0067] Dissolve 5g of polysulfone with a bromine content ...
Embodiment 3
[0075] Step 1: Synthesis of potassium salt of (4-carboxymethyl-3-oxa-hexafluorobutyl)-sulfo-(5-iodo-3-oxa-octafluoropentyl)sulfonylimide (ICF 2 CF 2 OCF 2 CF 2 SO 2 NKSO 2 CF 2 CF 2 OCF 2 COOCH 3 )
[0076] Add 50ml acetonitrile in 250ml there-necked flask, 0.01mol4-carboxy-3-oxa-hexafluorobutylsulfonamide (H 2 NSO 2 CF 2 CF 2 OCF 2 COOCH 3 ), 0.04mol of anhydrous potassium carbonate, mix uniformly and heat up to 60°C, add 0.01mol of 5-iodo-3-oxa-octafluoropentylsulfonyl fluoride monomer (ICF 2 CF 2 OCF 2 CF 2 SO 2 F) Reaction for 6h.
[0077] After the mixture was filtered, the mother liquor was rotary evaporated to remove acetonitrile, and the obtained yellow-white solid was recrystallized with deionized water to obtain the product ICF 2 CF 2 OCF 2 CF 2 SO 2 NKSO 2 CF 2 CF 2 OCF 2 COOCH 3 .
[0078] Step 2: Synthesis of fluorine-containing sulfonimide grafted polystyrene with carboxyl groups
[0079] Dissolve 5g of polystyrene with a bromine co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Li-ion conductivity | aaaaa | aaaaa |
| Li-ion conductivity | aaaaa | aaaaa |
| Li-ion conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com