A class of maleimide-type prodrugs with bioadhesive effects and their application in oral drug delivery
A maleimide-type, maleimidocaproyloxymethyl technology, applied in the new use field of prodrug preparations, can solve the side effects of heart, liver and kidney, inconvenient administration, and poor patient compliance and other problems to achieve the effect of improving oral bioavailability, increasing residence time, and improving permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
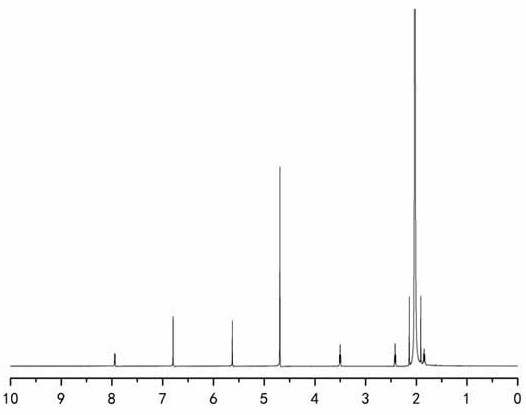
[0038] Preparation of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil
[0039] (a) Dissolve 9.806g (100mmol) of maleic anhydride and 13.118g (100mmol) of 6-aminocaproic acid in 100mL of glacial acetic acid, react at 120°C for 3-4h, remove organic reagents by rotary evaporation, use toluene with water, acetic acid After ethyl ester extraction, the organic layers were combined, anhydrous sodium sulfate was added and stirred to remove water, and column chromatography gave white intermediate (I).
[0040] (b) Dissolve 8.45 g (65 mmol) of 5-fluorouracil in a certain volume of 37% formaldehyde solution, react at 50°C for about 4 hours, and concentrate the reaction solution under reduced pressure to obtain a transparent liquid intermediate (II).
[0041] (c) 11.4g (54mmol) of 6-maleimidocaproic acid, 26.7g (70mmol) of HATU and 14.85mL (135mmol) of N-methylmorpholine were dissolved in 150mL of anhydrous acetonitrile, and the activation. Dissolve (Ⅲ) in acetonitrile and slowly transfe...
Embodiment 2
[0044] Determination of Equilibrium Solubility and Oil-Water Partition Coefficient
[0045] Add excess 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil to water, pH 1.2 PBS and PEG400 aqueous solutions with different contents, vortex for 5min, and then place in a constant temperature shaker at 37°C Shake in the box for 72h to make the dissolution reach equilibrium. The suspension was centrifuged at 12,000 r for 10 min, and the supernatant was filtered. After appropriate dilution, the drug concentration was measured by high performance liquid chromatography, and its equilibrium solubility in different media was calculated. The results are shown in Table 1.
[0046] Table 1
[0047]
[0048] Weigh respectively 5-fluorouracil and 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil in appropriate amount in water-saturated n-octanol (n-octanol-saturated water), respectively Mix it with n-octanol-saturated water (water-saturated n-octanol) in equal volume, shake in a shaker at 37°C fo...
Embodiment 3
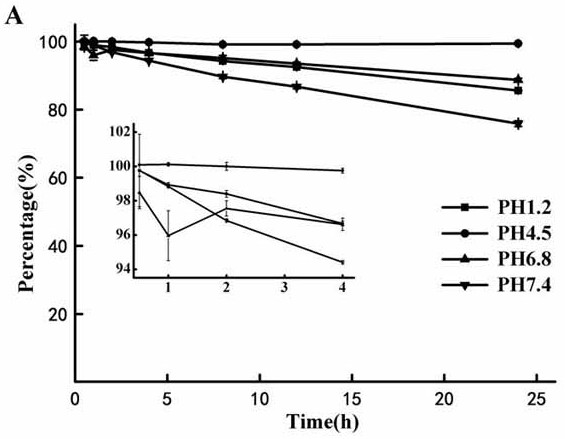
[0053] Study on the Stability of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil
[0054] Weigh an appropriate amount of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil, add appropriate amount of water to dissolve to prepare a stock solution with a concentration of about 200 μg / ml. Take an appropriate amount of the stock solution, and dilute it with phosphate buffer solution of pH 1.2, 4.5, 6.8 and 7.4 respectively, mix thoroughly, and perform triplicate. Put it in a constant temperature oscillator at 37°C by the classical constant temperature method, take samples at 0, 0.25, 0.5, 1, 2, 4, 8 and 12 hours, pass through a 0.45 μm filter membrane, and inject 10 μL into high performance liquid chromatography.
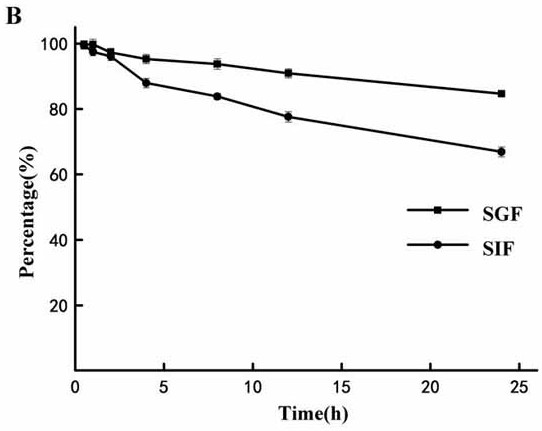
[0055] Artificial gastric fluid (SGF) and intestinal fluid (SIF) were prepared according to the Chinese Pharmacopoeia 2015 edition. Take an appropriate amount of stock solution, dilute and mix with artificial gastric juice and intestinal juice respectively, and make triplica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com