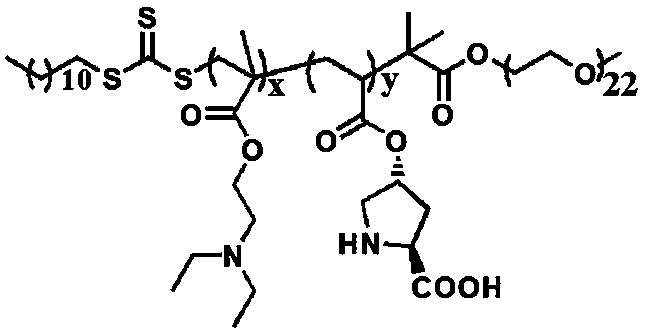
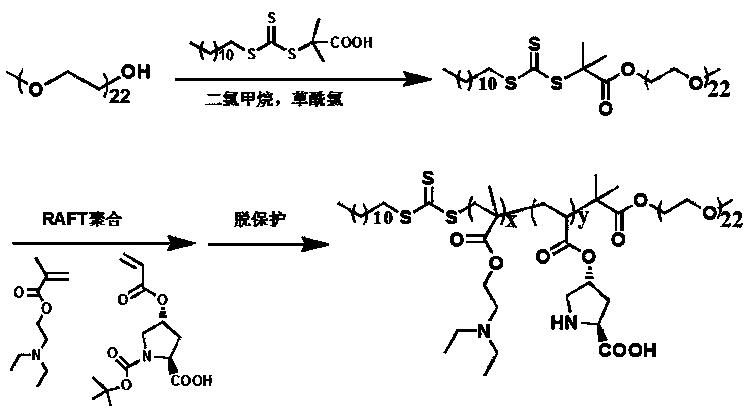
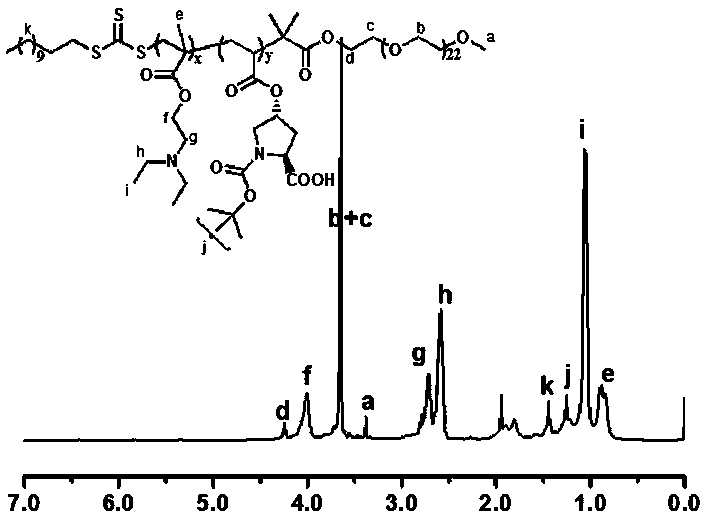
pH responsive block polymer immobilized with L-proline, and applications thereof
A block polymer and proline technology, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, asymmetric synthesis, etc., can solve the problems of inconvenient operation and energy consumption, and achieve improved The effect of catalytic yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Macromolecular Chain Transfer Agent Polyvinyl Alcohol Trithiocarbonate PEG-DDMAT
[0023] Put 1 g of 2-(dodecyltrithiocarbonate)-2-methylpropionic acid DDMAT in a dry round bottom flask, add 5 mL of dichloromethane to dissolve it, and add it dropwise under nitrogen atmosphere 5 mL of dichloromethane solution dissolved with 1 mL of oxalyl chloride was stirred sufficiently at room temperature for 5 hours. After the reaction, the system was dissolved in 5 mL of dichloromethane and rotary steamed 3 times to ensure removal of excess oxalyl chloride. Add 5 mL of dichloromethane solution containing 1 g of polyvinyl alcohol PEG-OH (1000) into the flask with the product from the previous step, and fully react with magnetic stirring at room temperature for 24 hours. The reaction solution is concentrated by rotary evaporation, and 20 mL of n-hexane is added to precipitate A yellow product was obtained. The product was dissolved in 5 mL of dichloromethane, concentra...
Embodiment 2
[0032] Preparation of Macromolecular Chain Transfer Agent Polyvinyl Alcohol Trithiocarbonate PEG-DDMAT
[0033] Put 2g of 2-(dodecyltrithiocarbonate)-2-methylpropionic acid DDMAT in a dry round bottom flask, add 10mL of dichloromethane to dissolve it, and add it dropwise under nitrogen atmosphere 10 mL of dichloromethane solution dissolved with 1 mL of oxalyl chloride was stirred fully at room temperature for 10 h. After the reaction, the system was dissolved in 10 mL of dichloromethane and rotary steamed 5 times to ensure the removal of excess oxalyl chloride. Add 10 mL of dichloromethane solution containing 2 g of polyvinyl alcohol PEG-OH (1000) into the flask with the product from the previous step, and fully react with magnetic stirring at room temperature for 24 hours. The reaction solution is concentrated by rotary evaporation, and 20 mL of n-hexane is added to precipitate A yellow product was obtained. The product was dissolved in 5 mL of dichloromethane, concentrated ...
Embodiment 3
[0039] Add the pH responsive block polymer PEG-b-P (DEAEMA-co-ProlA) of immobilized L-proline that the embodiment 1 that adds the amount of substrate p-nitrobenzaldehyde substance 10% as catalyst , 0.15mml p-nitrobenzaldehyde, 0.081 cyclohexanone and 1 mL H2O, add NaOH to adjust the pH to 7~8, react at room temperature for 24 hours, after the reaction is completed, feed CO into the system 2 , adjust the pH of the system to acidic, extract the product three times with diethyl ether (3×10mL), combine the organic phases, concentrate, and dry. The conversion rate is 92% and the anti / syn value is 92 / 8 according to the H NMR spectrum.
[0040] The catalytic system after product separation, add 0.15mml p-nitrobenzaldehyde, 0.081 cyclohexanone, and use H 2 O is calibrated to 1mL, adjust the pH to 7~8, react at room temperature for 24 hours, and pass CO into the system after the reaction is completed. 2 , adjust the pH of the system to acidity, extract the product three times with eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com