Hindered amine modified lignin as well as preparation method and application thereof
A technology of lignin and hindered amine, which is applied in the field of lignin materials, can solve the problems of chemically synthesized light stabilizers, low content of functional groups, serious molecular agglomeration and other problems in UV protection performance, and achieve rich variety and application range and short reaction time , The effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Dissolve 30g 2,2,6,6-tetramethyl-4-piperidinol and 10g parts of epichlorohydrin in 20g dimethylformamide, heat to 80℃, melt and mix uniformly, add 1g three Using boron fluoride catalyst, react at 80°C for 2 hours to obtain chlorotetramethylpiperidine intermediate;
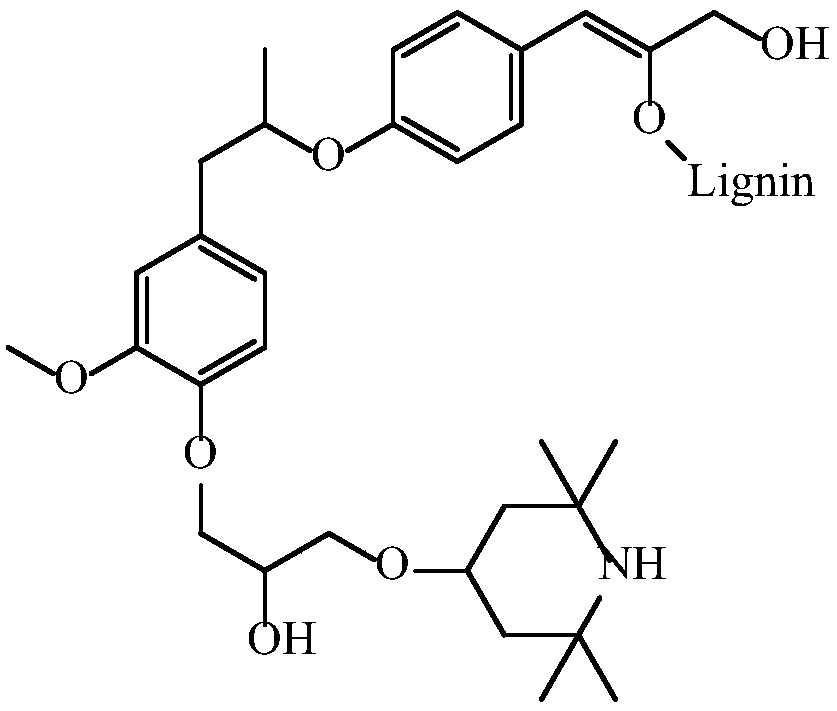
[0036] (2) Dissolve 100g of lignosulfonate in alkaline aqueous solution, prepare 20% solid content, adjust pH=10; then heat up to 90°C, add dropwise the chlorotetramethylpiperone prepared in step (1) The pyridine intermediate, after the completion of the dripping, the reaction is kept at 90℃ for 1 hour to obtain tetramethylpiperidine grafted modified lignin. See the structure diagram figure 1 .
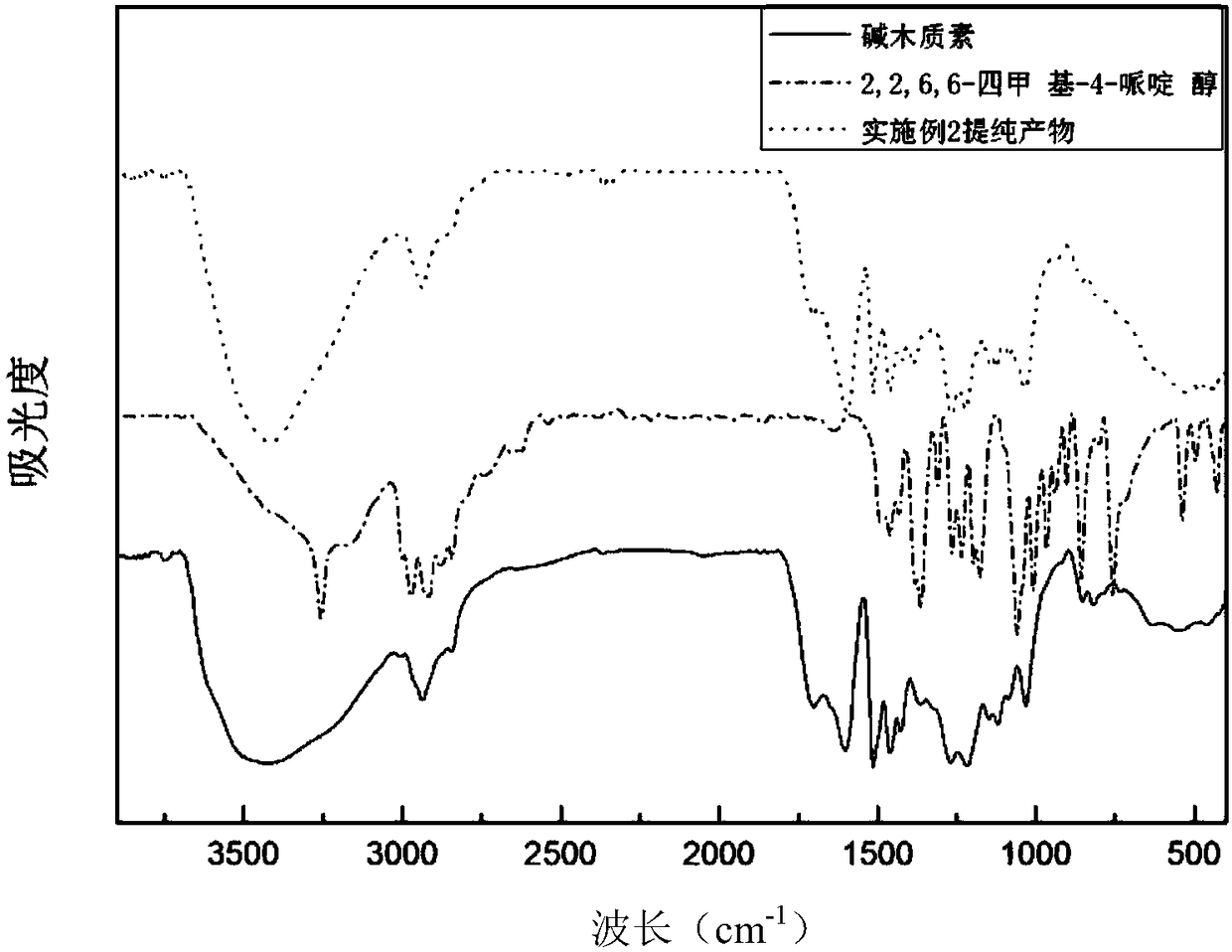
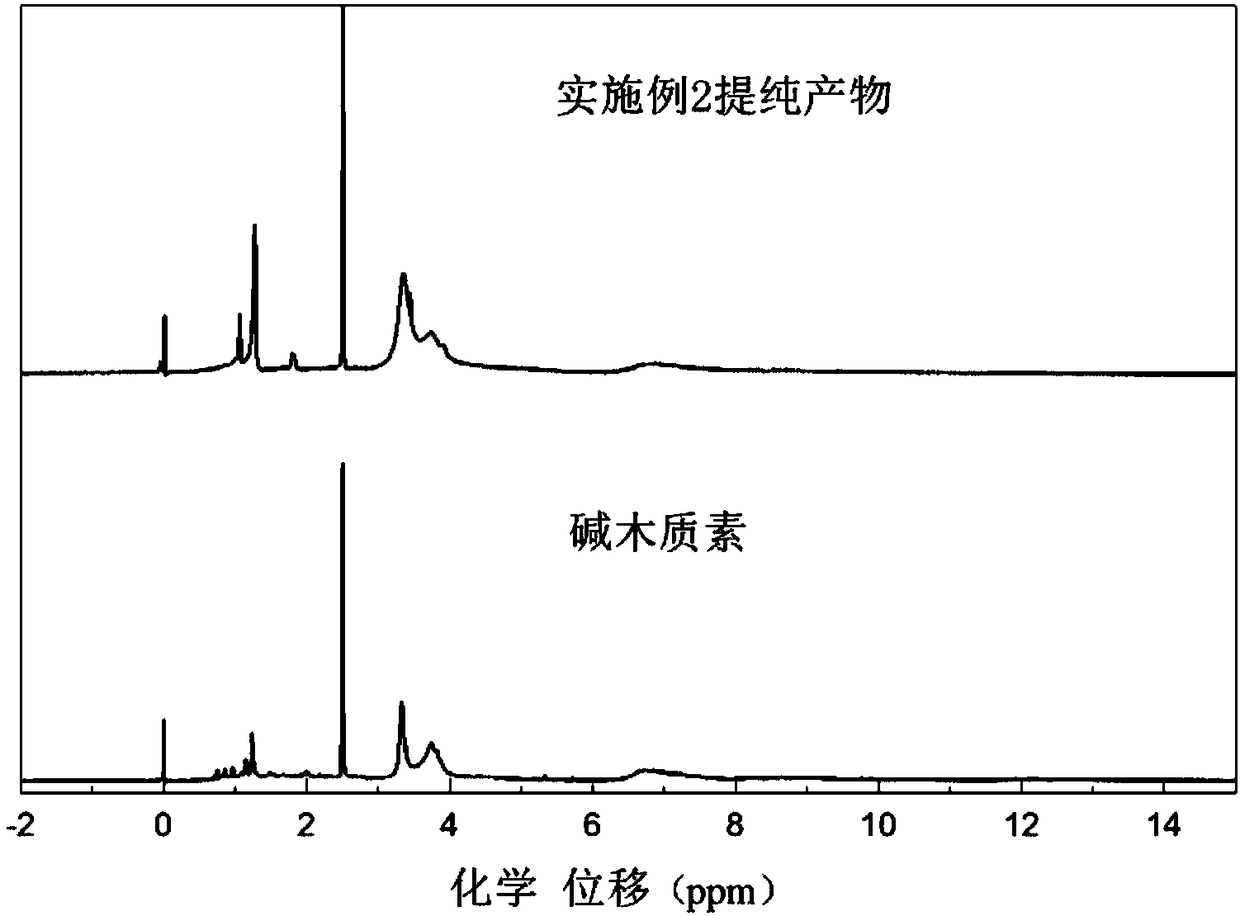
Embodiment 2
[0038] (1) Dissolve 50g 2,2,6,6-tetramethyl-4-piperidinol and 20g epichlorohydrin in 40g dimethylacetamide, heat to 50℃, melt and mix uniformly, add 1g chlorinated Aluminum catalyst, react at 50°C for 1.5 hours to obtain chlorotetramethylpiperidine intermediate;
[0039] (2) Dissolve 100g of alkali lignin in alkaline aqueous solution, prepare 25% solid content, adjust pH=12; then heat to 80°C, add dropwise the chlorotetramethylpiperidine prepared in step (1) After the dripping is finished, the reaction is kept at 80°C for 2 hours to obtain the tetramethylpiperidine grafted modified lignin.
Embodiment 3
[0041] (1) Dissolve 60g 2,2,6,6-tetramethyl-4-piperidinol and 20g epichlorohydrin in 20g tetrahydrofuran and 20g dioxane, heat to 50℃, melt and mix uniformly, add 1.5g With tin tetrachloride catalyst, react at 55°C for 2 hours at elevated temperature to obtain chlorotetramethylpiperidine intermediate;
[0042] (2) Dissolve 100g of solvent-based lignin in alkaline aqueous solution, prepare 40% solid content, adjust pH=14; then heat to 70°C, add dropwise the chlorotetramethylpiperidine prepared in step (1) After the completion of the dripping, the intermediate is incubated and reacted at 70°C for 3 hours to obtain tetramethylpiperidine grafted modified lignin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com