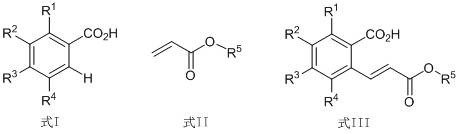
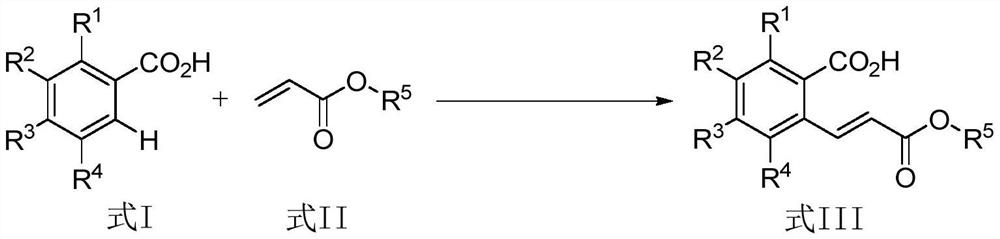
A method for cross dehydrogenation coupling of aromatic carboxylic acid and α, β-unsaturated ester
A cross-dehydrogenation coupling, aromatic carboxylic acid technology, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds and other directions, can solve the problems of harsh reaction conditions, many reaction steps, no reports, etc. The effect of mild reaction conditions, low temperature requirements and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
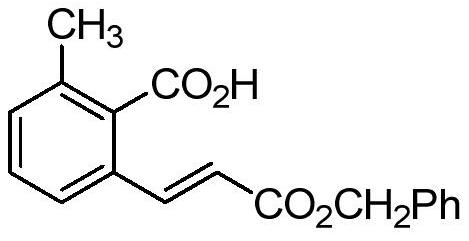
[0018] Preparation of (E)-2-methyl-6-(3-benzyloxy-3-oxo-1-propenyl)benzoic acid with the following structural formula
[0019]
[0020] Add 13.6 mg (0.1 mmol) o-toluic acid, 30 μL (0.2 mmol) benzyl acrylate, and 3.1 mg (0.005 mmol) pentamethylcyclopentadienyl rhodium dichloride dimer to a 10 mL pressure-resistant reaction tube , 25.4mg (0.2mmol) of silver fluoride, 0.6mL of dioxane, stirred and reacted at 60°C for 3 hours under the protection of argon, cooled to room temperature after the reaction, and filtered through a column chromatography silica gel column (ethyl acetate lotion), remove the catalyst, and separate by thin layer chromatography (with petroleum ether, ethyl acetate, acetic acid volume ratio of 100:20:1 mixed solution as the developer), to obtain (E)-2-methyl-6- (3-benzyloxy-3-oxo-1-propenyl)benzoic acid, its yield is 64%, and the structural characterization data are as follows:
[0021] 1 H NMR (600MHz, CDCl 3 ):δ[ppm]=8.52(s,1H),8.00(d,J=15.8Hz,1H),7.47...
Embodiment 2
[0025] Preparation of (E)-2-ethyl-6-(3-benzyloxy-3-oxo-1-propenyl)benzoic acid with the following structural formula
[0026]
[0027] The o-toluic acid used in Example 1 is replaced with an equimolar amount of o-ethylbenzoic acid, and the other steps are the same as in Example 1 to obtain (E)-2-ethyl-6-(3-benzyloxy -3-oxo-1-propenyl)benzoic acid, its yield is 69%, and the structural characterization data are as follows:
[0028] 1 H NMR (400MHz, CDCl 3 ):δ[ppm]=9.47(s,1H),8.00(d,J=15.8Hz,1H),7.50(d,J=7.7Hz,1H),7.43-7.28(m,7H),6.48(d ,J=15.8Hz,1H),5.25(s,2H),2.78(q,J=7.5Hz,2H),1.27(t,J=7.5Hz,3H).
[0029] 13 C NMR (100MHz, CDCl 3 ): δ[ppm]=173.2, 166.6, 142.7, 142.2, 135.8, 133.0, 132.1, 130.6, 130.1, 128.5, 128.2, 128.1, 124.1, 120.3, 66.5, 26.9, 15.6.
[0030] HRMS(ESI)m / z:C 19 h 18 o 4 ,[M+Na] +, The theoretical value is 333.1103; the measured value is 333.1105.
Embodiment 3
[0032] Preparation of (E)-2-phenyl-6-(3-benzyloxy-3-oxo-1-propenyl)benzoic acid of the following structural formula
[0033]
[0034] The o-toluic acid used in Example 1 is replaced with 2-phenylbenzoic acid in equimolar amounts, and other steps are the same as in Example 1 to obtain (E)-2-phenyl-6-(3-benzyloxy Base-3-oxo-1-propenyl)benzoic acid, its yield is 65%, and the structural characterization data are as follows:
[0035] 1 H NMR (400MHz, CDCl 3 ):δ[ppm]=9.10(s,1H),8.02(d,J=15.8Hz,1H),7.64(d,J=7.7Hz,1H),7.49(t,J=7.8Hz,1H), 7.44-7.28(m,11H),6.52(d,J=15.8Hz,1H),5.24(s,2H).
[0036] 13 C NMR (150MHz, CDCl 3 ): δ[ppm]=172.9,166.4 142.2,140.9,139.8,135.8,133.1,132.4,131.6,129.9,128.5,128.4,128.3,128.2,128.2,127.8,125.3,121.0,66.5.
[0037] HRMS(ESI)m / z:C 23 h 18 o 4 ,[M+Na] + , The theoretical value is 381.1103; the measured value is 381.1101.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com