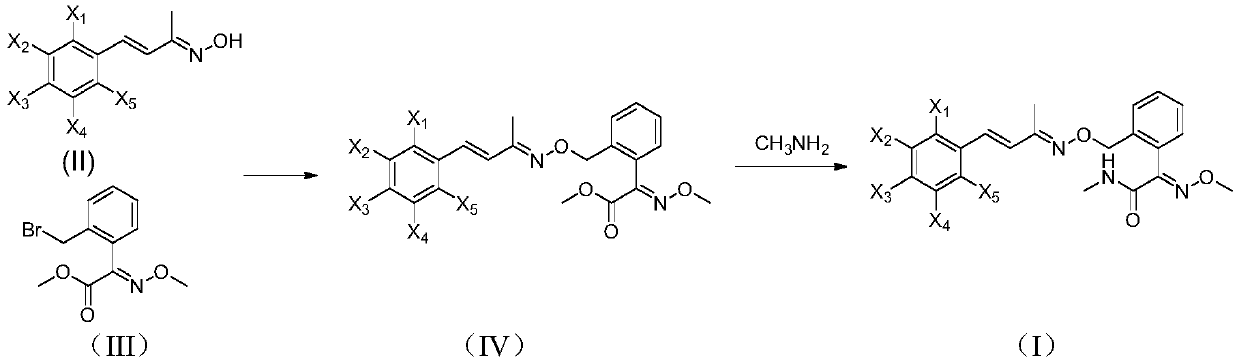
A kind of method for preparing unsaturated oxime ether compound
A technology of ether compounds and compounds, applied in oxime preparation, organic chemistry, etc., can solve the problems of complex post-processing and restrictions on industrial production, and achieve the effects of simple post-processing, easy industrial production, and high bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
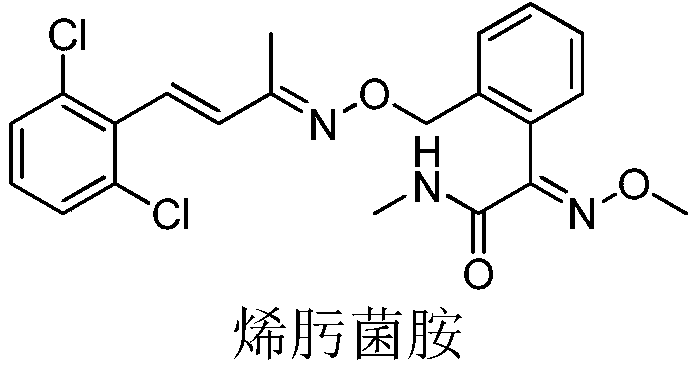
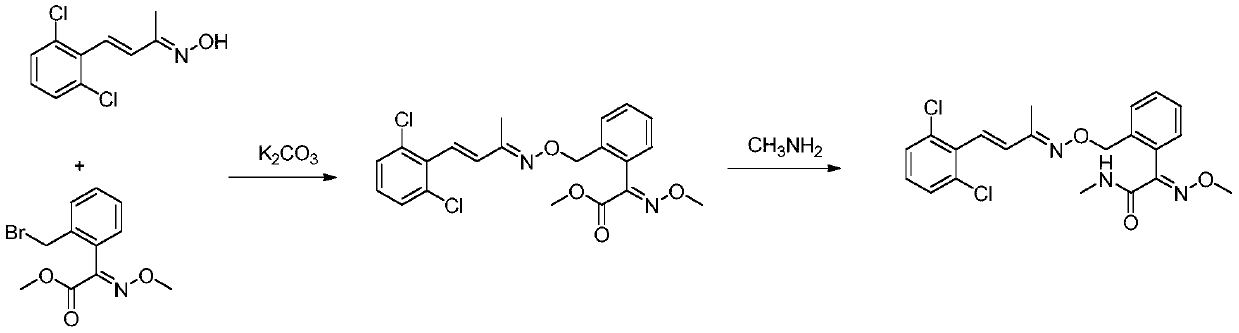
[0024] Methoxyimino-N-methyl-2-[[[[[[4-(2,6-dimethyl)phenyl]-3-buten-2-yl]imino]oxy]methyl ] Synthesis of phenylacetamide
[0025]
[0026] Add (E)-2-(bromomethyl)-α-methoxyiminophenylacetic acid methyl ester (75.5g, 0.2mol), acetone (390g), 2,6-dichlorostyryl Methyl ketoxime (46.1 g, 0.20 mol) and potassium carbonate (44.6 g, 0.32 mol) were heated up to 58°C and kept at reflux for 5 hours. After the reaction is over, the water bath is heated at 80°C under normal pressure to recover acetone until there is basically no distillate, and the precipitation is stopped. Add dichloromethane (350mL) and 150g of water, stir until completely dissolved, then let stand for stratification, separate the organic phase and wash it with water (150g) once, after separating the washed organic phase, heat it in a water bath at 70°C and distill it to the basic Continue depressurizing for 10 min after no fractions, then stop, add methanol (122g) after cooling down slightly, heat up and reflux u...
Embodiment 2
[0030] Methoxyimino-N-methyl-2-[[[[[[4-(2,6-dimethyl)phenyl]-3-buten-2-yl]imino]oxy]methyl ] Synthesis of phenylacetamide (solvent butanone)
[0031]
[0032] Add (E)-2-(bromomethyl)-α-methoxyiminophenylacetic acid methyl ester (75.5g, 0.2mol), methyl ethyl ketone (350g), 2,6-dichlorostyrene to the reaction flask in sequence Methyl ketoxime (46.1g, 0.2mol) and potassium carbonate (41.4g, 0.30mol) were heated to 60°C and kept for 4h. After the reaction is over, the butanone is recovered under reduced pressure in a water bath until there is basically no distillate, and the precipitation is stopped. Add dichloromethane (350mL) and 150g of water, stir until completely dissolved, then let stand to separate layers, separate the organic phase and wash it once with water (150g), after separating the washed organic phase, heat it in a water bath at 70°C and distill it to the basic Continue depressurizing for 10 min after no fractions, then stop, add methanol (122g) after cooling d...
Embodiment 3
[0036] Methoxyimino-N-methyl-2-[[[[[[4-(2,6-dimethyl)phenyl]-3-buten-2-yl]imino]oxy]methyl ] Synthesis of phenylacetamide
[0037]
[0038]Add (E)-2-(bromomethyl)-α-methoxyiminophenylacetic acid methyl ester (75.5g, 0.2mol), acetone (390g), 2,6-dichlorostyryl Methyl ketoxime (46.1 g, 0.20 mol) and cesium carbonate (97.7 g, 0.30 mol) were heated up to 58° C. and refluxed for 4 hours. After the reaction is over, the water bath is heated at 80°C under normal pressure to recover acetone until there is basically no distillate, and the precipitation is stopped. Add dichloromethane (350mL) and 150g of water, stir until completely dissolved, then let stand for stratification, separate the organic phase and wash it with water (150g) once, after separating the washed organic phase, heat it in a water bath at 70°C and distill it to the basic Continue depressurizing for 10 minutes to stop after no fraction, add methanol (122g) after cooling down slightly, heat up and reflux until the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com